Method for synthesizing p-hydroxyacetophenone
A technology for p-hydroxyacetophenone and a synthesis method, which is applied in the field of synthesis of p-hydroxyacetophenone, can solve the problems of complicated post-treatment, unfriendly environment, etc., and achieves the effects of being environmentally friendly, simple in method, and high in industrialized production value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
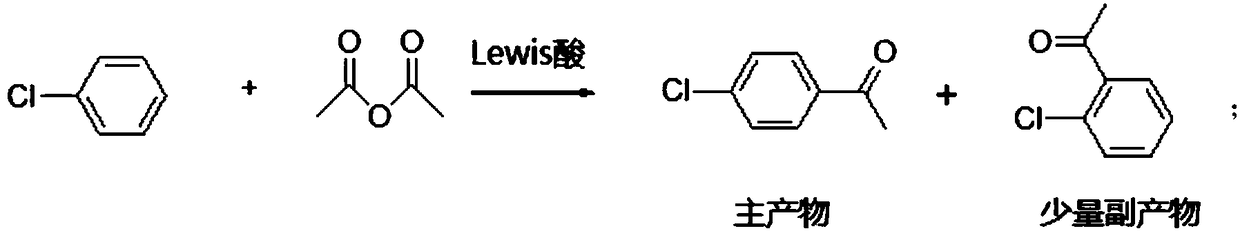
[0036] Chlorobenzene (1125.6g) was added in a 2L four-necked flask, and aluminum trichloride (294g, 2.2mol, 1.1q) was added in batches, then the temperature was raised to 50°C, and acetic anhydride (204g, 2mol, 1.0eq) was added slowly Slowly drop it into the reactor, control the temperature of the reaction solution at 50°C to 55°C, and finish the drop in about 1.5hr. The temperature was later raised to ~75°C for 4hrs. The reaction is over. Slowly pour the reaction solution into a 1 kg ice-water bath, control the internal temperature -5 ~ 5 ° C, after adding the feed solution, warm it up to room temperature, and separate the liquids. The organic phase reclaimed 900 g of chlorobenzene, and then rectified to obtain 272 g of p-chloroacetophenone, with a yield of 88% and 18.6 g of o-chloroacetophenone, with a yield of 6% (the yield was calculated based on the chlorobenzene consumed by the reaction).
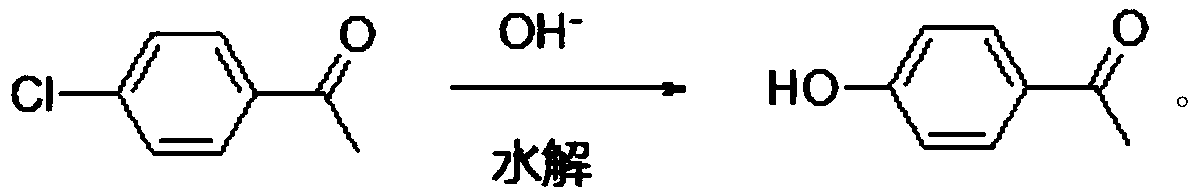
[0037] In a 2L autoclave, add 1000g of 30% sodium hydroxide solution, and then ...
Embodiment 2
[0039] Chlorobenzene (1125.6g) was added in a 5L four-necked flask, and aluminum trichloride (647g, 4.84mol, 1.2q) was added in batches, then the temperature was raised to 50°C, and acetic anhydride (408g, 4mol, 1.0eq) was slowly Slowly drop it into the reactor, control the temperature of the reaction solution at 55°C to 60°C, and finish the drop in about 1.5hr. The temperature was later raised to ~70 °C for 4 hrs. The reaction is over. Slowly pour the reaction solution into a stirred 2.5 kg 1% hydrochloric acid bath, control the internal temperature -5 ~ 5 ℃, add the feed solution, rise to room temperature, and separate the liquids. The organic phase reclaims 448g of chlorobenzene, and then rectifies to obtain 533g of p-chloroacetophenone, with a yield of 86.2% and 35g of o-chloroacetophenone, with a yield of 5.6% (the yield is calculated by the chlorobenzene consumed by the reaction).
[0040] In a 2L autoclave, 1000 g of 50% sodium hydroxide solution was added, and then 3...
Embodiment 3
[0042] Chlorobenzene (900g) was added to a 2L four-necked flask, and aluminum chloride (387.5g, 2.9mol, 1.45q) was added in batches, then the temperature was raised to 50°C, and acetic anhydride (204g, 2mol, 1.0eq) was slowly Slowly drop it into the reactor, control the temperature of the reaction solution at 55°C to 60°C, and finish the drop in about 1.5hr. The temperature was later raised to ~70°C for 5hrs. The reaction is over. Slowly pour the reaction solution into 1.5 kg of 1% ice dilute hydrochloric acid bath, raise to room temperature, and separate the layers. The organic phase reclaims chlorobenzene 674g, rectification obtains p-chloroacetophenone 259g, yield 84% and o-chloroacetophenone 18.6g, yield 6% (calculate yield with the chlorobenzene consumed by reaction).
[0043]In a 2L autoclave, 1000 g of 45% potassium hydroxide solution was added, and then 309.2 g of p-chloroacetophenone was added, and the mixture was sealed and heated to an inner temperature of 160°C. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com