A method for separating and analyzing bedaquiline optical isomers
A technology of optical isomers and bedaquiline, applied in the field of liquid chromatography, can solve problems such as difficult quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Apparatus and conditions
[0035]Chromatographic column: Daicel CHIRALPAKZWIX(+) (4.6*150mm, 3μm);
[0036]Mobile phase: 2.6mL diethylamine, 1.9mL formic acid to 1000mL methanol;
[0037]Column temperature: 12℃;
[0038]Wavelength: 226nm;
[0039]Flow rate: 0.1mL / min;
[0040]Injection volume: 1μL.
[0041]Experimental steps
[0042](1S,2R)-Betaquinoline stock solution: Weigh 10mg of (1S,2R)-Betaquinoline, place in a 10mL measuring flask, dilute to the mark with methanol, shake well, and then pipette 1mL into 100mL volume In the bottle, dilute with methanol to a constant volume.
[0043]Test solution: Weigh (1R, 2S)-Bedaquinoline 10mg, accurately weigh it, put it in a 20mL measuring flask, add methanol to dissolve and dilute to the mark, shake well.
[0044]System suitability solution: Weigh 10 mg of (1R, 2S)-Betaquinoline into a 10mL volumetric flask, pipette 1.0 mL of (1S, 2R)-Betaquinoline stock solution into this volumetric flask, add methanol to dissolve and dilute To the mark, shake well.
[0045](1R, 2...
Embodiment 2
[0050]Apparatus and conditions
[0051]Chromatographic column: Daicel CHIRALPAKZWIX(+) (4.6*150mm, 3μm);
[0052]Mobile phase: 2.6mL diethylamine, 1.9mL formic acid to 1000mL methanol;
[0053]Column temperature: 12℃;
[0054]Wavelength: 226nm;
[0055]Flow rate: 0.08mL / min;
[0056]Injection volume: 1μL.
[0057]The experimental procedure is the same as in Example 1.
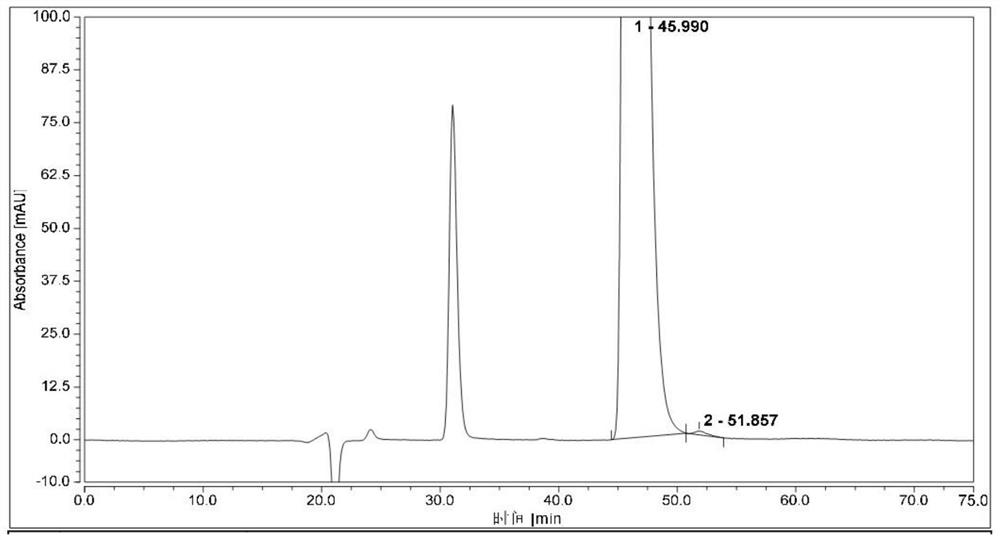
[0058]Measure 1μL of the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram. The resolution of (1S,2R)-bedaquinoline peak and (1R,2S)-bedaquinoline peak is greater than 1.5.
[0059]Take 1μL each of the test solution and (1R,2S)-bedaquinoline self-control solution, perform HPLC analysis under the above conditions, and record the chromatogram. The content of (1S, 2R)-bedaquinoline was calculated according to the self-control method with correction factor, and the content was 0.2%.
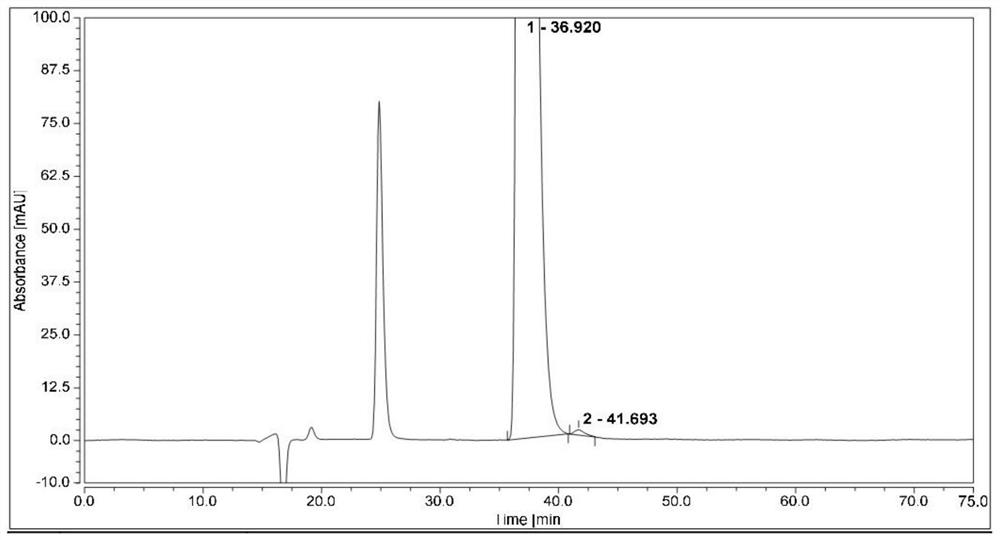
[0060]The results are attachedfigure 2 , The peak 1 in the figure is the isomer (1R,2S)-bedaquinoline, and the peak 2 is...
Embodiment 3
[0062]Apparatus and conditions
[0063]Chromatographic column: Daicel CHIRALPAKZWIX(+) (4.6*150mm, 3μm);
[0064]Mobile phase: 2.6mL diethylamine, 1.9mL formic acid to 1000mL methanol;
[0065]Column temperature: 12℃;
[0066]Wavelength: 226nm;
[0067]Flow rate: 0.09mL / min;
[0068]Injection volume: 1μL.
[0069]The experimental procedure is the same as in Example 1.
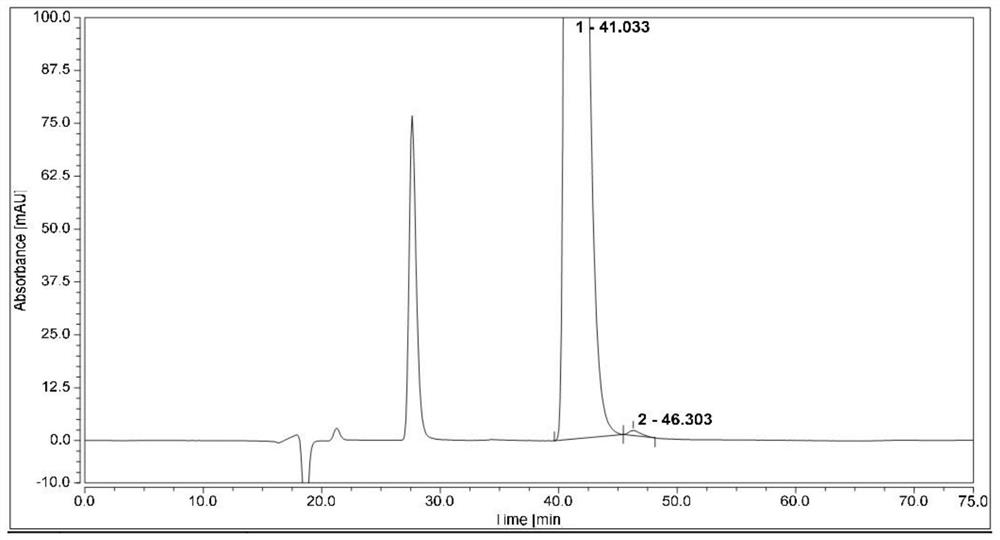
[0070]Measure 1μL of the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram. The resolution of (1S,2R)-bedaquinoline peak and (1R,2S)-bedaquinoline peak is greater than 1.5.
[0071]Take 1μL each of the test solution and (1R,2S)-bedaquinoline self-control solution, perform HPLC analysis under the above conditions, and record the chromatogram. The content of (1S, 2R)-bedaquinoline was calculated according to the self-control method with correction factor, and the content was 0.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com