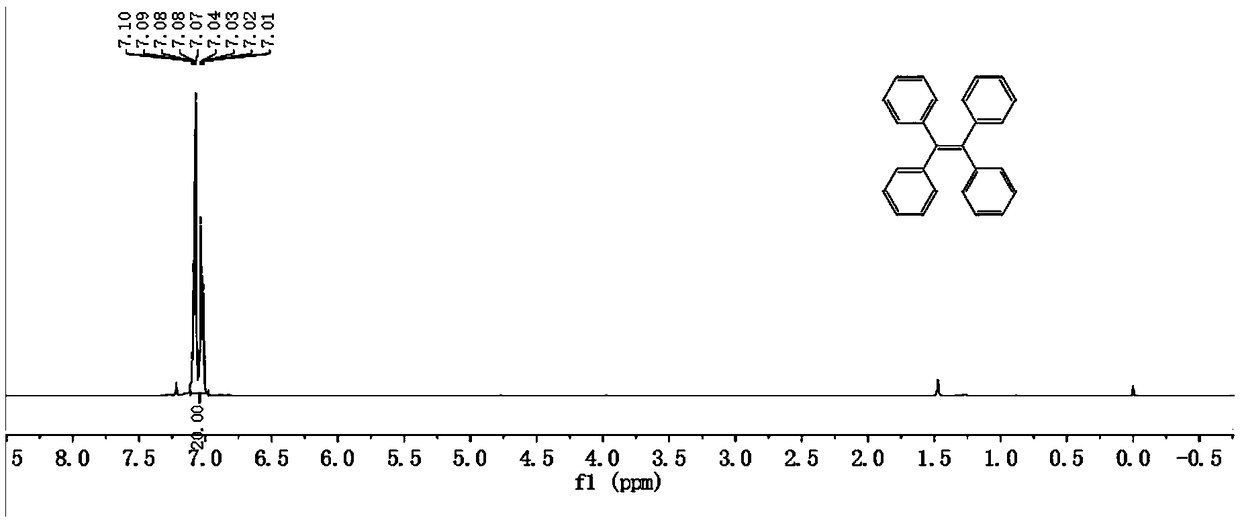
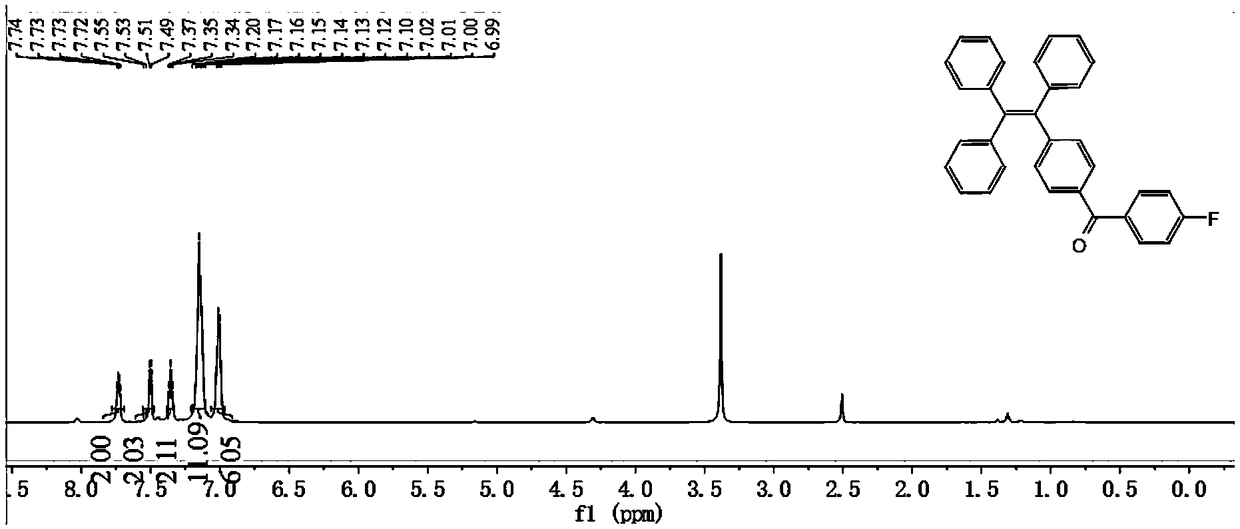
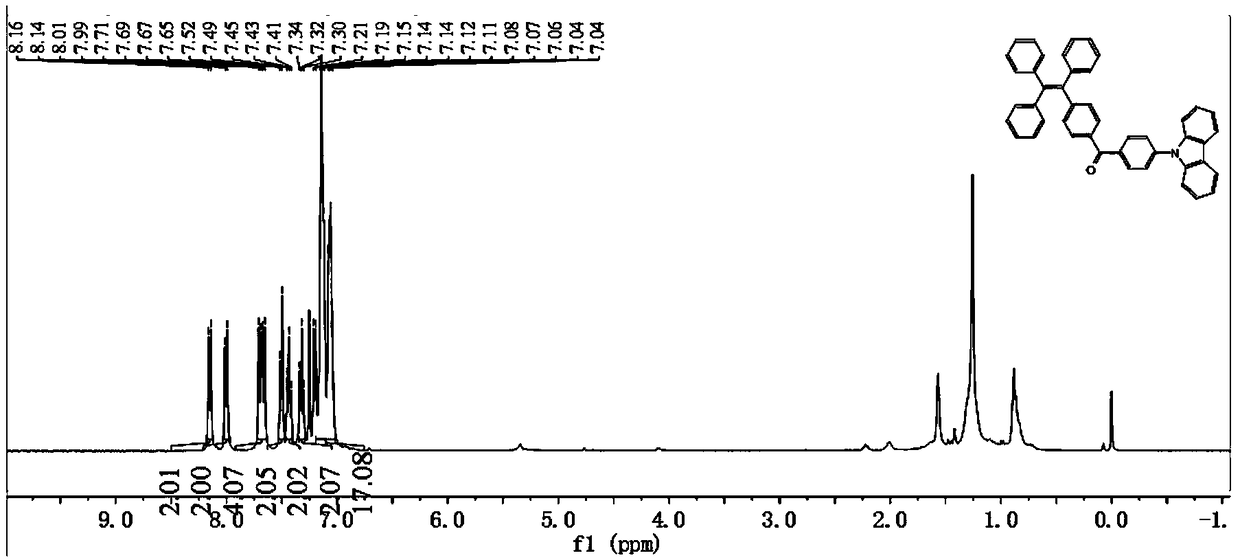
Tetraphenylethylene-benzophenone-carbazole derivative, crystal and preparation method and application thereof
A technology of carbazole derivatives and tetraphenylethylene, which is applied in the field of organic light-emitting materials, can solve the problems of restricting large-scale applications, reducing luminous intensity, and difficult preparation, etc., and achieves suppression of exciton annihilation, good luminous performance, and high luminous brightness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] A tetraphenylethylene-benzophenone-carbazole derivative prepared by the following method:
[0059] S1. Preparation of intermediate Ⅰ
[0060] A. Weigh 1.82g of benzophenone and 1.315g of zinc powder in a 250mL round bottom flask, add 150mL of anhydrous tetrahydrofuran to dissolve, and obtain a mixed solution;
[0061] B. Magnetically stir the mixed solution obtained in step A under the protection of nitrogen, cool to 0°C, inject 4.4mL of titanium tetrachloride, and stir in an ice bath for 30min (generally, the temperature of ice bath stirring can be -10~ 0 ℃, in embodiment 1 is-10 ℃), warm to room temperature, reflux 20h at 90 ℃, along with the carrying out of reaction, the color of reaction solution deepens gradually, turns black from light brown at the beginning, and reaction solution is opaque and turbid state;
[0062] C. After the reaction solution was cooled to room temperature, the reaction was quenched with 1:1 dilute hydrochloric acid (20 mL hydrochloric acid...
Embodiment 2
[0078] A tetraphenylethylene-benzophenone-carbazole derivative, the preparation method thereof is compared with the preparation method of Example 1, the difference is that the temperature of the ice-bath stirring in step S1. of Example 2 is -5 ° C, Time 60min, reflux heating time is 18h;
[0079] Other raw material consumption and operating steps are consistent with embodiment 1.
Embodiment 3
[0081] A kind of tetraphenylethylene-benzophenone-carbazole derivative, its preparation method is compared with the preparation method of embodiment 1, difference is, the time of Friedel-Crafts reaction in the step S2. of embodiment 3 is 5h; Step The temperature of heating and refluxing in S3. is 110°C, and the time is 12h;
[0082] Other raw material consumption and operating steps are consistent with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Emission wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com