Synthetic method of flucloxacillin sodium
A technology of flucloxacillin sodium and a synthesis method, which is applied in the field of antibiotic synthesis, can solve problems such as seriousness, equipment corrosion, and storage difficulties, and achieves the effects of reducing production costs, facilitating production operations, and having high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
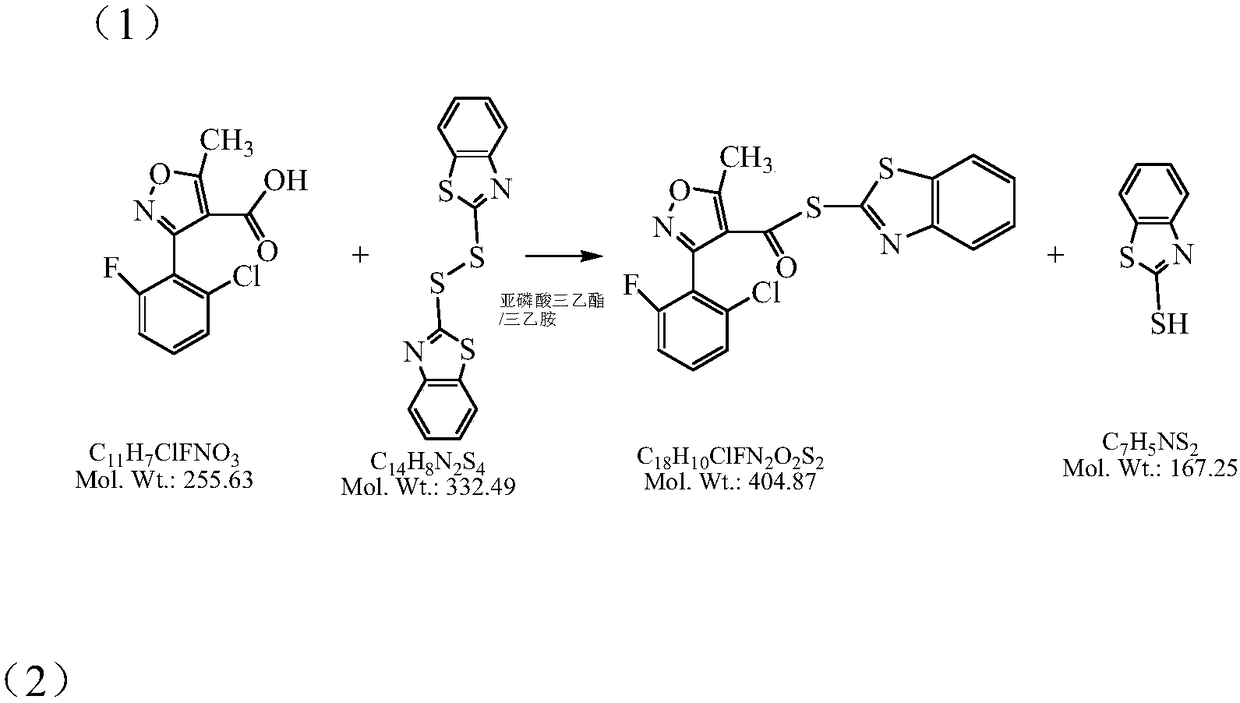
[0053] (1) Add 9L of dichloromethane, 3.0kg of 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylic acid, and 4.3kg of DM into a clean enamel reaction kettle.
[0054] (2) Start stirring and temperature control, and maintain the temperature of the material at 5-15°C.
[0055] (3) Add 1.3kg of triethylamine and 2.3Kg of triethyl phosphite mixture dropwise, and react for 3 hours.
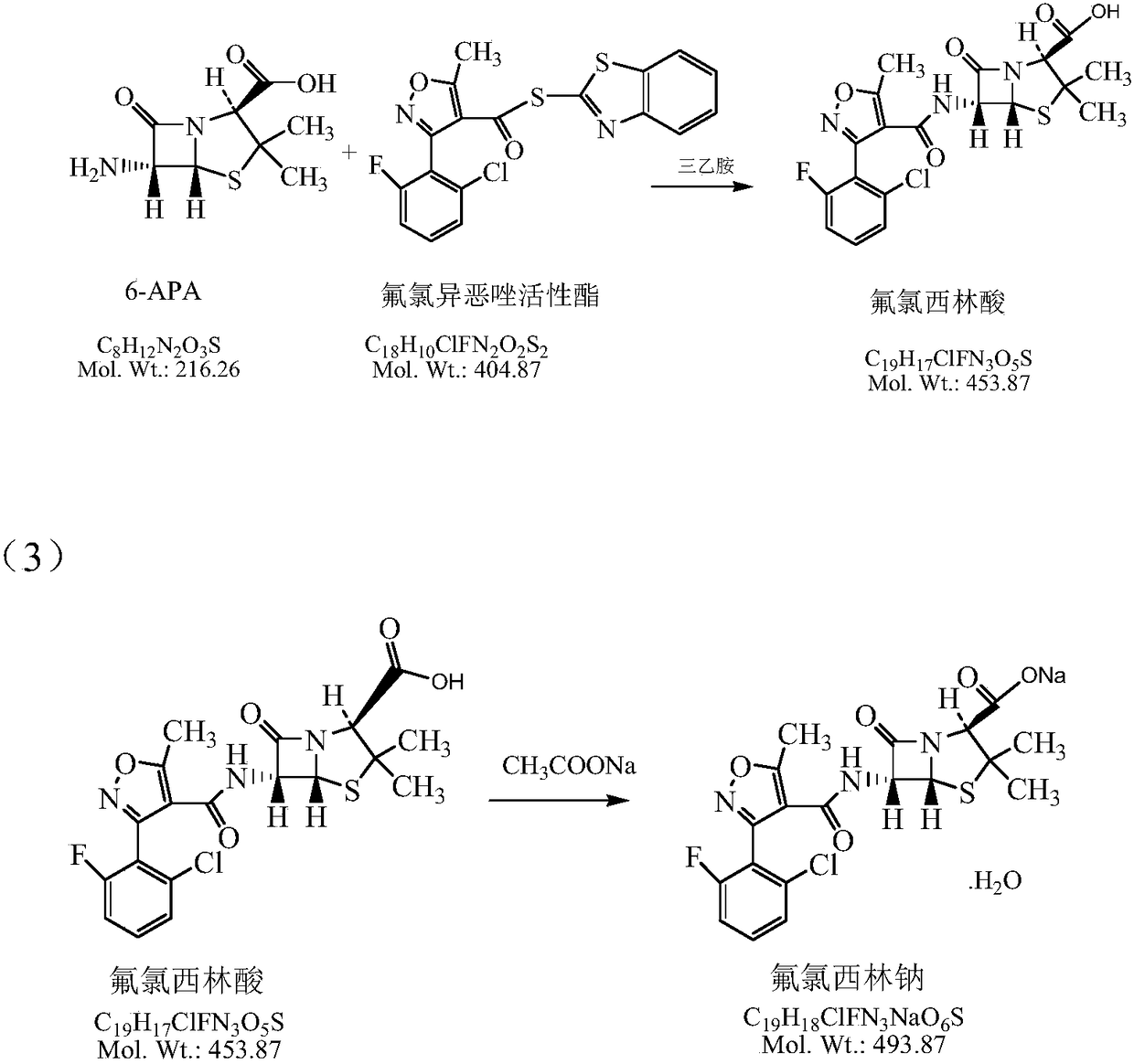
[0056] (4) After the reaction, add 4L of purified water and 2.5kg of 6-APA,
[0057] (5) Continue to add 0.5Kg of triethylamine dropwise, maintain the temperature at 8-15°C, and keep the pH value at 6.5-8.5. Sampling and detection of 6-APA was less than 0.5%.
[0058] (6) Add dilute sulfuric acid dropwise, adjust the pH value to 5.0-6.5, stir for 5-10 minutes, and let stand to separate layers.
[0059] (7) Add 4 L of dichloromethane to the aqueous phase for extraction, stir for 5-10 minutes, and let stand to separate layers.
[0060] (8) Water phase collection Add 9 L of methyl acetate, cont...
Embodiment 2
[0069] (1) Add 10L of dichloromethane, 3.0kg of 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylic acid, and 5.0kg of DM into a clean enamel reaction kettle.
[0070] (2) Start stirring and temperature control materials to maintain a temperature of 5 ~ 15 ℃,
[0071] (3) Add 1.5 kg of triethylamine and 2.5 kg of triethyl phosphite mixture dropwise, and react for 3 to 5 hours.
[0072] (4) After the reaction, add 5L of purified water and 2.8kg of 6-APA,
[0073] (5) Continue to drop 0.8Kg of triethylamine to maintain the temperature at 8-15°C and the pH at 6.5-8.5. Sampling and detection of 6-APA was less than 0.5%.
[0074] (6) Add dilute sulfuric acid dropwise, adjust the pH to 5.0-6.5, stir for 5-10 minutes, and let stand to separate layers.
[0075] (7) Add 5 L of dichloromethane to the aqueous phase for extraction, stir for 5-10 minutes, and let stand to separate layers.
[0076] (8) Add 10 L of ethyl acetate for water phase collection, continue to add dilute s...
Embodiment 3
[0085] (1) Add 9L of dichloromethane, 3.0kg of 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylic acid, and 4.0kg of DM into a clean enamel reaction kettle.
[0086] (2) Start stirring and temperature control materials to maintain a temperature of 5 ~ 15 ℃,
[0087] (3) Add 1.2kg of triethylamine and 2.0Kg of triethyl phosphite mixture dropwise, and react for 3 to 5 hours.
[0088] (4) After the reaction, add 5L of purified water and 2.8kg of 6-APA,
[0089] (5) Continue to drop 0.5Kg of triethylamine to maintain the temperature at 8-15°C and the pH at 6.5-8.5. Sampling and detection of 6-APA was less than 0.5%.
[0090] (6) Add dilute sulfuric acid dropwise, adjust the pH to 5.0-6.5, stir for 5-10 minutes, and let stand to separate layers.
[0091] (7) Add 4 L of dichloromethane to the aqueous phase for extraction, stir for 5-10 minutes, and let stand to separate layers.
[0092] (8) Collect the aqueous phase and add 9 L of ethyl butyl acetate, continue to drop di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com