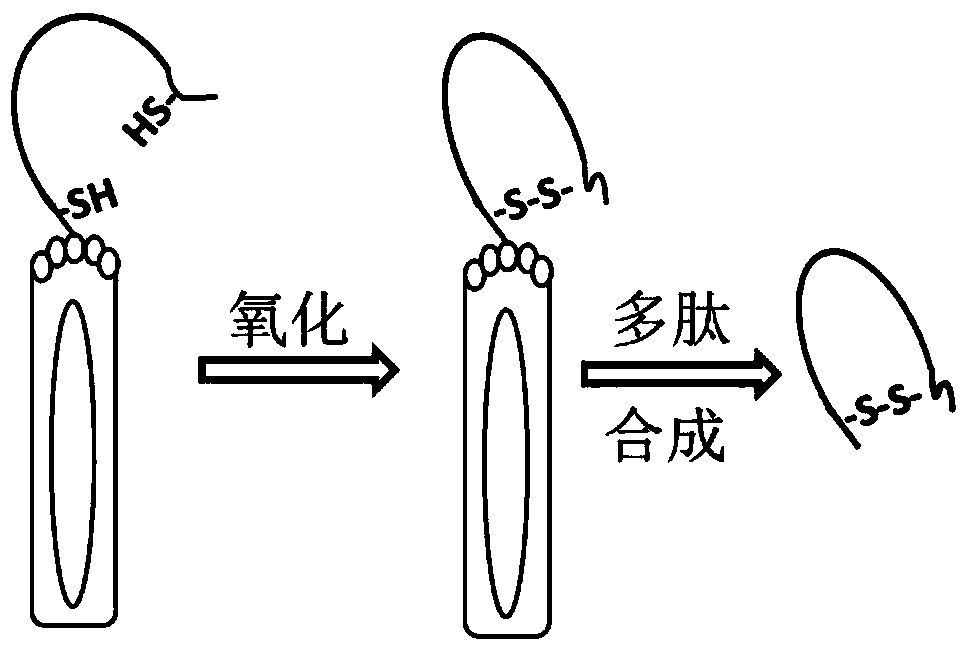
Ring type polypeptide simulating antigenic epitope of aflatoxin B1 (AFB1), and detection kit of aflatoxin B1 (AFB1)
An aflatoxin and cyclic polypeptide technology, applied in the field of immunoanalytical chemistry, can solve the problems of large batch impact and high cost of competitive antigen preparation, and achieve the effect of improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Specific panning and amplification of phage-displayed polypeptides
[0048] Panning of Phage Displayed Specific Peptides
[0049] Coat 5 wells on a 96-well ELISA plate, the first well is coated with 100 μg / mL antibody, the remaining 4 wells are coated with 3% BSA, and coated overnight at 4°C, the next day, add 200 μL 0.05% PBST to wash the plate 3 times; Block the ELISA plate with 1% BSA, incubate at 25°C for 2 hours, wash the plate 3 times; add 1 μL of phage display cyclic heptapeptide library to well 1, incubate for 1 hour, add 500ppb AFB1 for competitive elution after washing the plate, incubate at 25°C for 1 hour, and Transfer the eluate to wells 2, 3, 4, and 5 at 25 μL / well. After incubation for 1 hour, collect the supernatant, which is the eluate after panning. Use the upper plate method to determine the concentration of phage in the eluate. Titer. The antibody concentrations of the second round of panning and the third round of panning were halved seq...
Embodiment 2
[0052] Example 2 Identification and sequencing of specific polypeptides
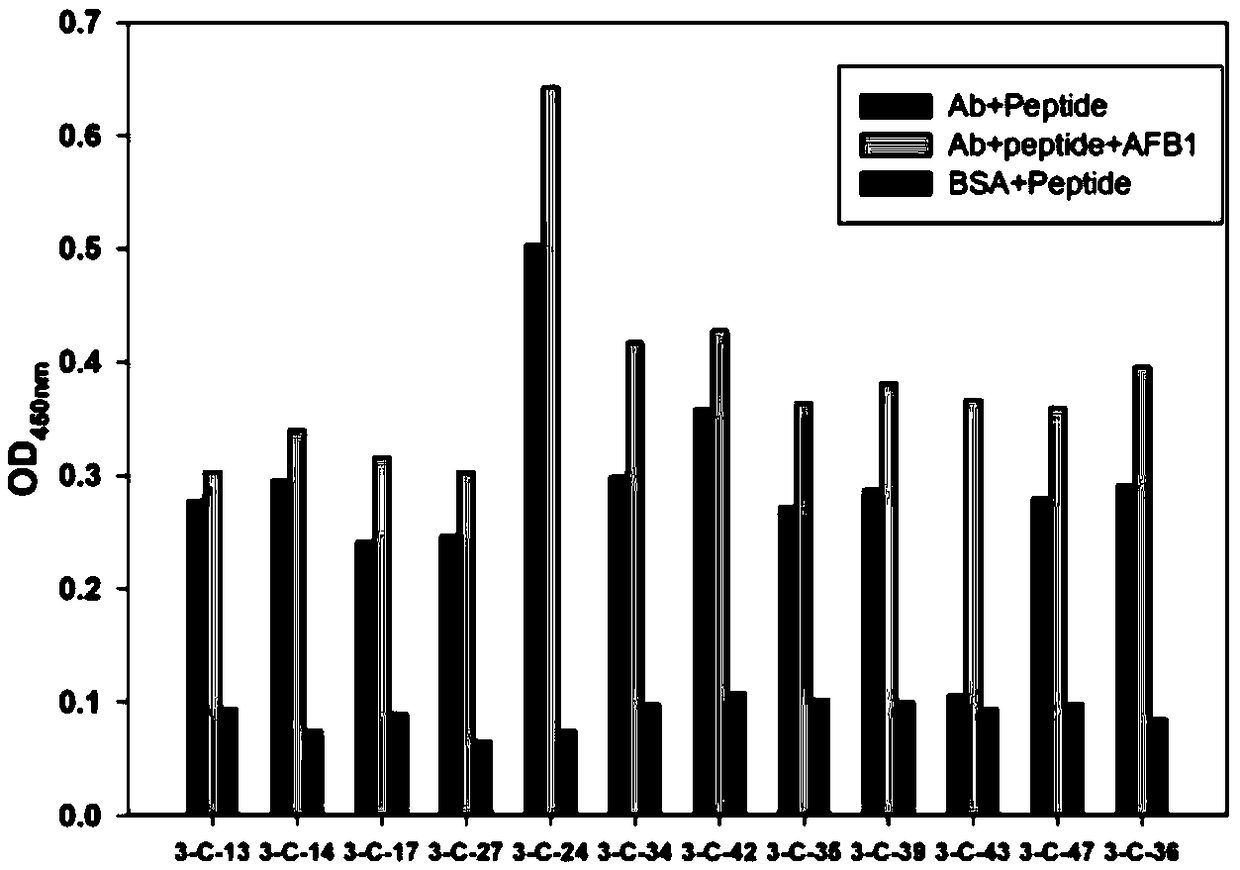
[0053] Pick a single clone from the plate of the eluted phage in the third round of the above-mentioned determination, and use the Phage ELISA method to measure the ability of the phage-displayed polypeptide clone to mimic the AFB1 antigen epitope and bind to the antibody. The specific operation is: inoculate the picked phage plaque In 2 mL of LB medium (containing 1:100 log prophase E. coli ER2738), shake culture at 37°C for 6 h, centrifuge at 10,000 rpm for 10 min, and collect the supernatant. Each clone was measured in 3 wells. Well 1 and well 2 were coated with 100 μL (10 μg / mL) of the antibody used for screening, and well 3 was coated with 1% BSA as a blank control. After washing with PBST, 3% skimmed milk was added to block for 2 h ( 300 μL / well), add 50 μL 500ppb AFB1 and 50 μL plaque culture supernatant mixture to well 1, add 50 μL 10% methanol PBS and 50 μL plaque culture supernatant mixture to ...
Embodiment 3
[0055] Example 3 Synthesis of biotin-labeled cyclic polypeptides
[0056] Biotin reacts with -NH2 in the cyclic polypeptide. There are two free -NH2 in the cyclic polypeptide sequence, one is at the N-terminal and the other is at the R group of lysine, so the cyclic polypeptide and biological Elements are connected at a ratio of 1:2. Weigh 2mg of cyclic polypeptide, add 50μL DMF and 2mL PBS buffer to dissolve the polypeptide;
[0057] Weigh 4.6 mg of biotin, add 50 μL LDMF to dissolve, take 22 μL of dissolved biotin and add dropwise to the dissolved polypeptide, ice bath for 2 hours and then dialyze for 24 hours to obtain biotin-labeled cyclic polypeptide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com