An efficient and stable mn 4+ Doped fluoride light-emitting material and preparation method thereof
A technology of luminescent materials and fluorides, which is applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems of insufficient high temperature and humidity resistance, and achieve the effects of high temperature stability and humidity resistance, short time, and easy process control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: K 2 TiF 6 :Mn 4+ Phosphor powder preparation
[0040] 0.4000g K 2 MnF 6 Dissolve in 6mL hydrofluoric acid (49wt.%) solution, stir 5 minutes to obtain orange-yellow transparent solution, then 5.1850g K 2 TiF 6 Add the powder into the solution, continue stirring at room temperature for 30 minutes, stop stirring and filter with filter paper, then wash with acetone to remove any residual HF, and dry to obtain K 2 TiF 6 :Mn 4+ powder. The excitation and emission spectra, fluorescence quantum yield and absorption efficiency of the product were measured by a FLS980 (Edinburgh Instrument) fluorescence spectrometer.
Embodiment 2
[0041] Example 2: Modified K 2 TiF 6 :Mn 4+ Phosphor powder preparation
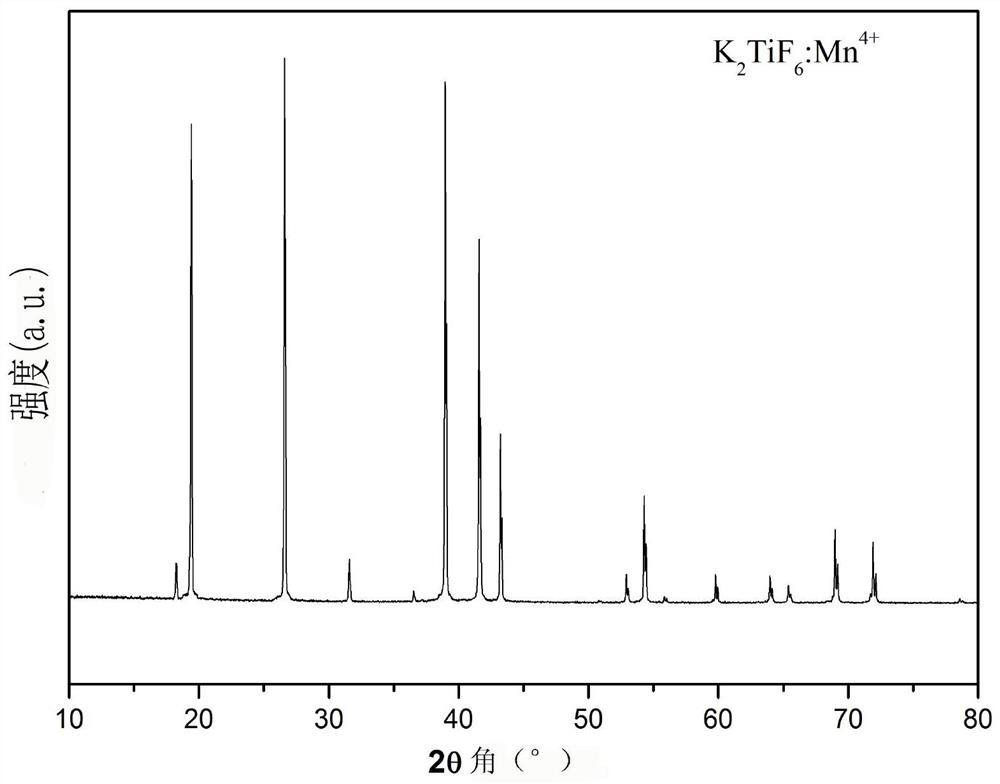
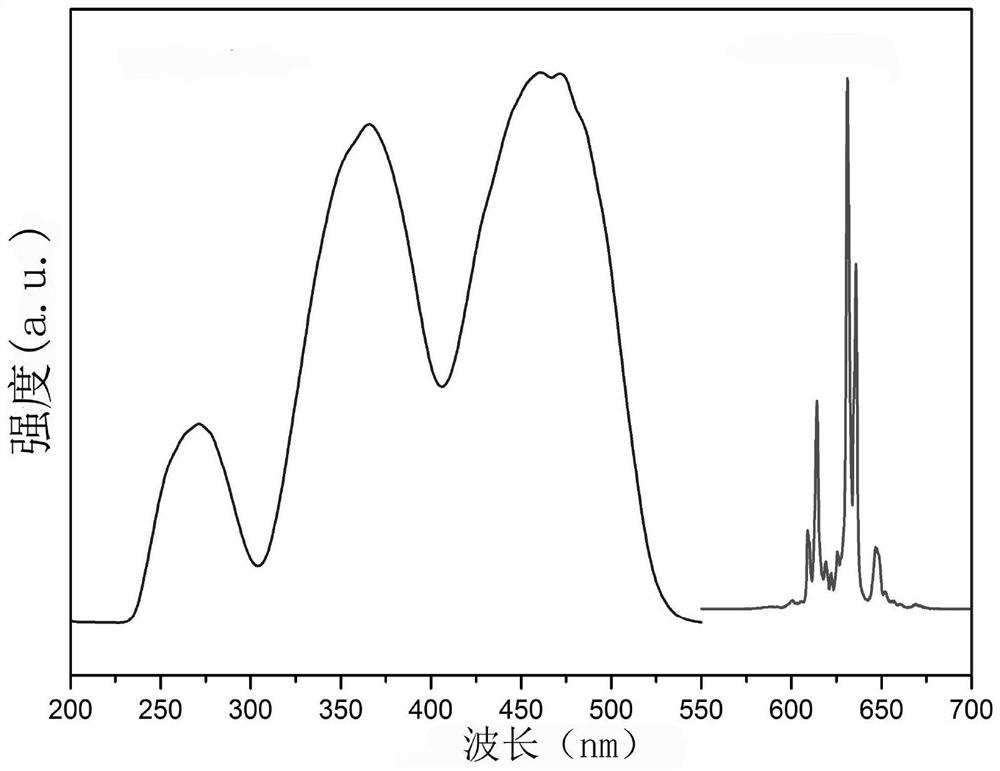
[0042] By adding K to 10 ml of 49% HF solution 2 TiF 6 Compound, until no longer dissolved (about 1.7000g, at room temperature), filter the undissolved K 2 TiF 6 , get K 2 TiF 6 Saturated solution in 49% HF solution. Then add this saturated solution to the K 2 TiF 6 :Mn 4+ In the phosphor container, keep stirring at room temperature for 30min. Then perform vacuum filtration; repeat the above stirring and soaking steps twice, and finally filter and wash with acetone several times to remove residual HF, and dry at 70°C for 2 hours to obtain phosphor. X-ray powder diffraction showed that the product was still in the hexagonal phase K 2 TiF 6 structure( figure 1 ); The excitation and emission spectra and the internal fluorescence quantum yield of the product were measured by a FLS980 type (Edinburgh Instrument) fluorescence spectrometer, figure 2 For its excitation and emission spectra, Tabl...
Embodiment 3
[0043] Example 3: Modified K 2 TiF 6 :Mn 4+ Phosphor powder preparation
[0044] By adding K to 10 ml of 49% HF solution 2 TiF 6 compound, the solution was heated to 70°C and stirred continuously until it was no longer dissolved (about 2.3000g), and the undissolved K was filtered 2 TiF 6 , get K 2 TiF 6 Saturated solution in 49% HF solution at 70 °C. Then add this saturated solution to the K 2 TiF 6 :Mn 4+ In the phosphor container, the temperature was slowly lowered at a cooling rate of 0.5 °C / min, and the stirring was continued until room temperature. Then perform vacuum filtration, wash with acetone several times to remove residual HF, and dry at 70°C for 2 hours to obtain phosphor. Table 1 shows the important physicochemical and optical performance parameters of the prepared phosphors, including Mn 4+ The doping concentration, the preparation raw material ratio and the fluorescence quantum yield and absorption efficiency of the sample.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com