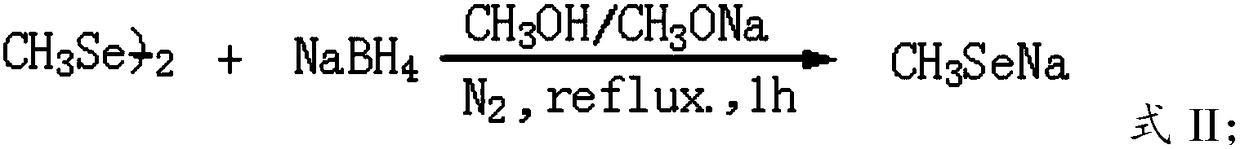L-selenomethionine preparation method
A technology of selenomethionine and hydrochloric acid solution, applied in the field of fine chemical synthesis, can solve the problems of product needing to be split, serious pollution, complicated treatment process, etc., and achieve the effects of mild reaction conditions, single product and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
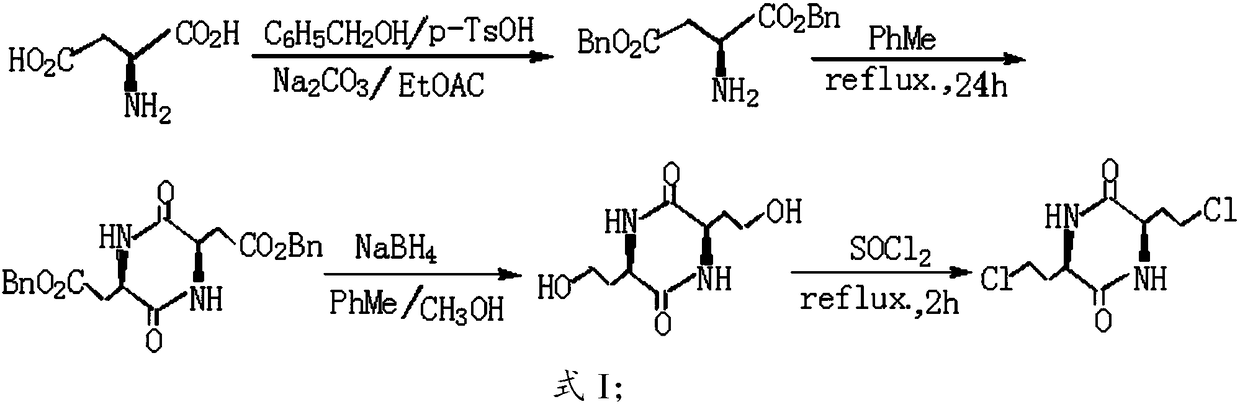
[0040] The invention provides a kind of preparation method of L-selenomethionine, comprises the following steps:
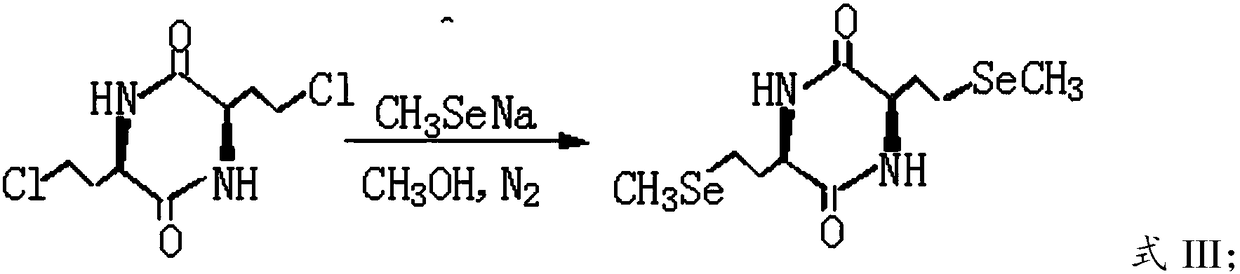
[0041] A) Carry out nucleophilic substitution reaction between sodium methylselenide and 3,6-bis-(2-chloroethyl)-2,5-diketopiperazine to obtain 3,6-bis-(2-methylselenoethyl) )-2,5-diketopiperazine;
[0042] B) hydrolyzing the 3,6-bis-(2-methylselenoethyl)-2,5-diketopiperazine obtained in the step A) in a hydrochloric acid solution to obtain L-selenomethionine.
[0043]The present invention has no special restrictions on the source of the 3,6-bis-(2-chloroethyl)-2,5-diketopiperazine, preferably according to the synthesis process route in the present invention, with L-aspartic acid The acid is used as the starting material, and it is prepared by esterification, cyclization and reduction, and chlorination in sequence.
[0044] Concrete preparation steps are as follows:
[0045] Esterifying L-aspartic acid and alcohol under the action of a catalyst to obtain an est...
Embodiment 1
[0084] Preparation of L-selenomethionine using 3,6-bis-(2-chloroethyl)-2,5-diketopiperazine:
[0085] 1. Preparation of 3,6-bis-(2-chloroethyl)-2,5-diketopiperazine
[0086] 1) Esterification
[0087] In a round-bottomed flask equipped with an electric stirrer, a water separator, and a reflux condenser, add 66.5 grams of L-aspartic acid, p-TsOH.H 2 O 90 grams, benzyl alcohol 150 mL and 500 mL benzene. Heat and reflux in an oil bath until no water evaporates, stop the reaction, evaporate most of the benzene, after cooling, add 500mL of a 1:1 mixture of diethyl ether and petroleum ether, put it in the refrigerator overnight, white crystals precipitate, filter, and use A 1:1 mixture of ethanol and ether was recrystallized to obtain 202 g of L-aspartate dibenzyl p-toluenesulfonate with a yield of 82.6%.
[0088] Melting point m.p. is 150~152℃, CHC1 3 :CH 3 OH=7:1R f =0.68, specific rotation [α] D 20=+0.9°(C=1,CH 3 OH).
[0089] 2) Cyclization and reduction
[0090] In a...
Embodiment 2
[0104] Preparation of L-selenomethionine using 3,6-bis-(2-chloroethyl)-2,5-diketopiperazine:
[0105] 1. The preparation method of 3,6-bis-(2-chloroethyl)-2,5-diketopiperazine is the same as in Example 1.
[0106] 2. Preparation of L-selenomethionine
[0107] With stirrer, N 2 Add 400mL of methanol and 2.0g of metallic sodium to the three-necked flask of the airway, and after the sodium dissolves, add 4.2g of sodium borohydride, 2 Under protection, 8.3 g of dimethyl diselenide was added dropwise, and after the drop was completed, the reaction was stirred at 58-60°C for 1 hour;
[0108] Then lower the temperature to 50°C, add 9.6 g of 3,6-bis-(2-chloroethyl)-2,5-diketopiperazine, heat and reflux and stir for 3 hours to 3,6-bis-(2-chloroethyl) The reaction of -2,5-diketopiperazine was complete (TLC detection), filtered while it was hot, and the filtrate was placed in the refrigerator overnight, and a white solid was precipitated, filtered with suction, washed with water, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com