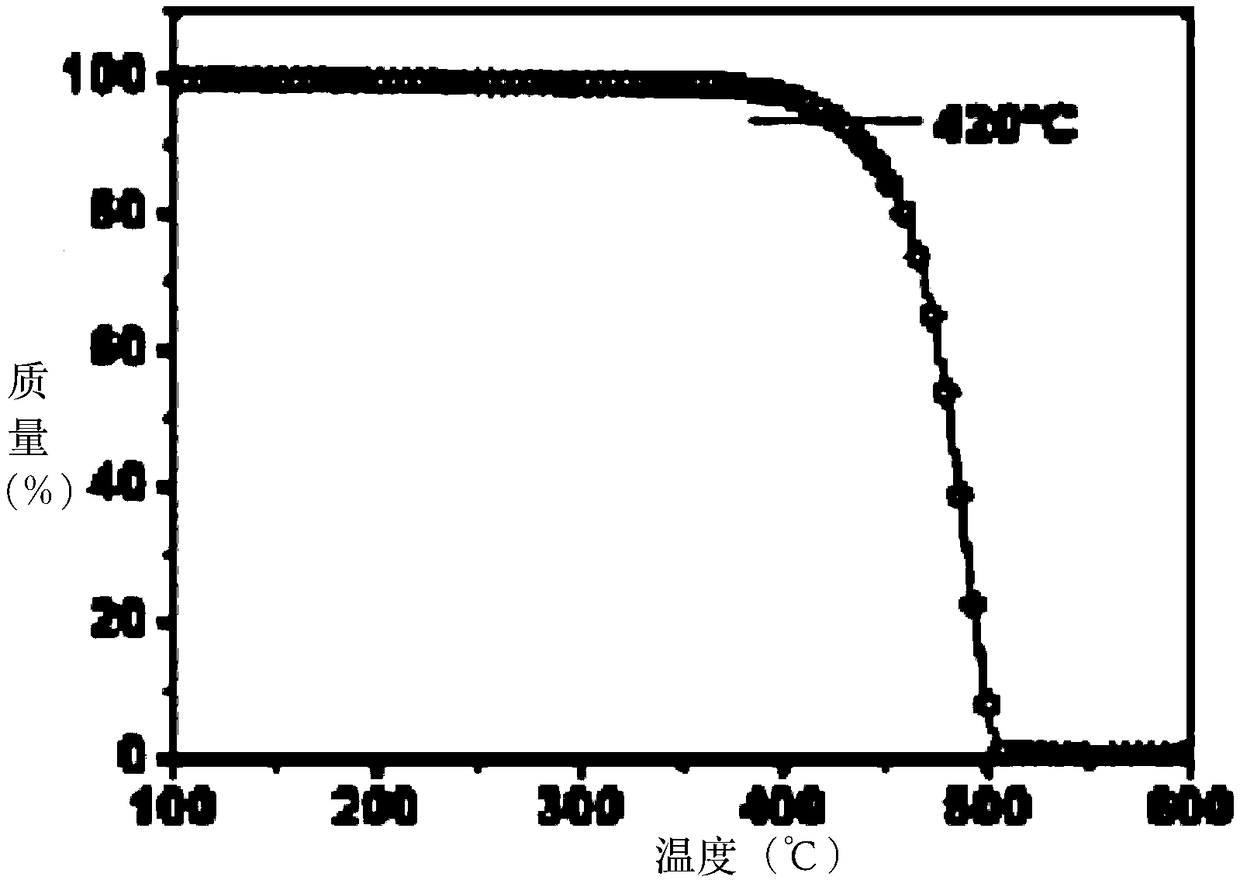
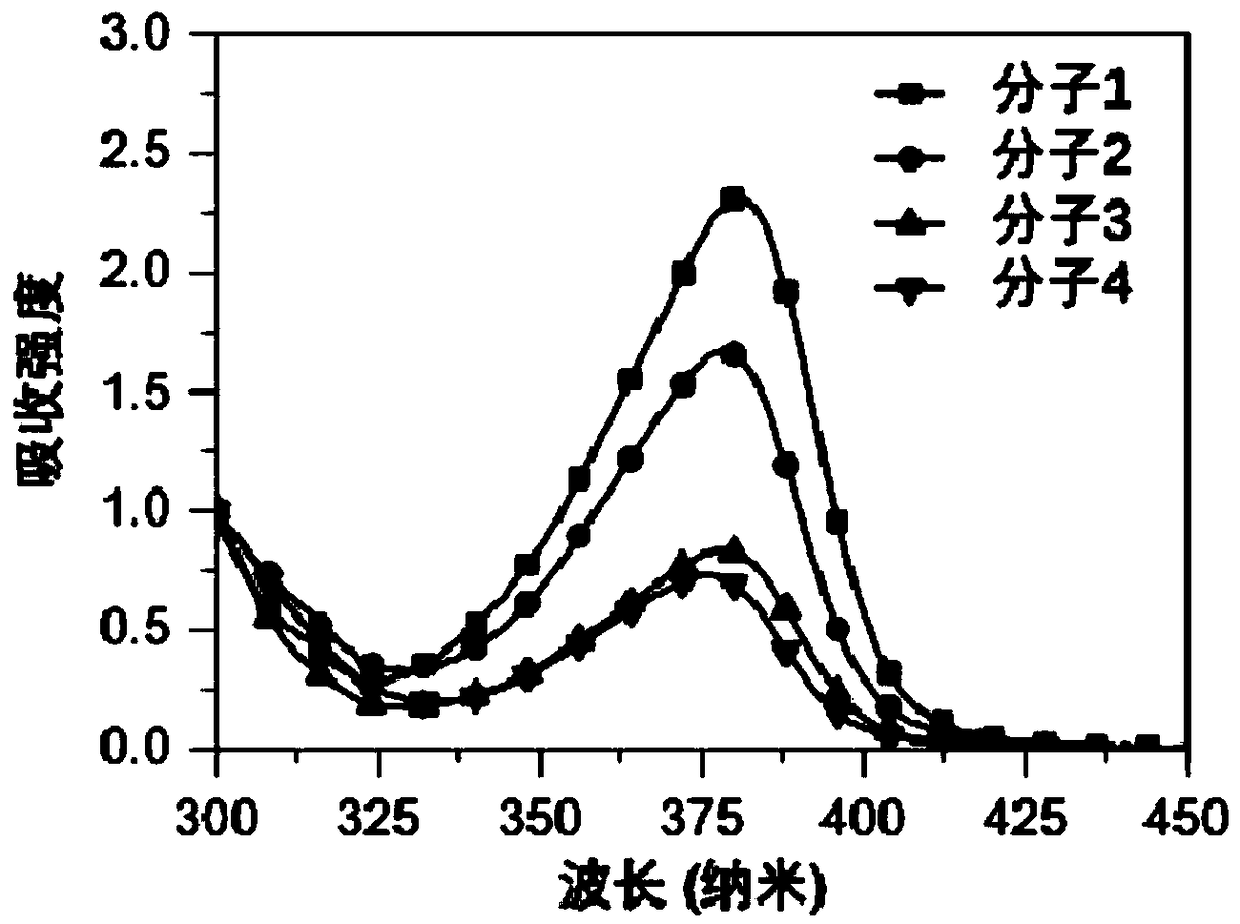
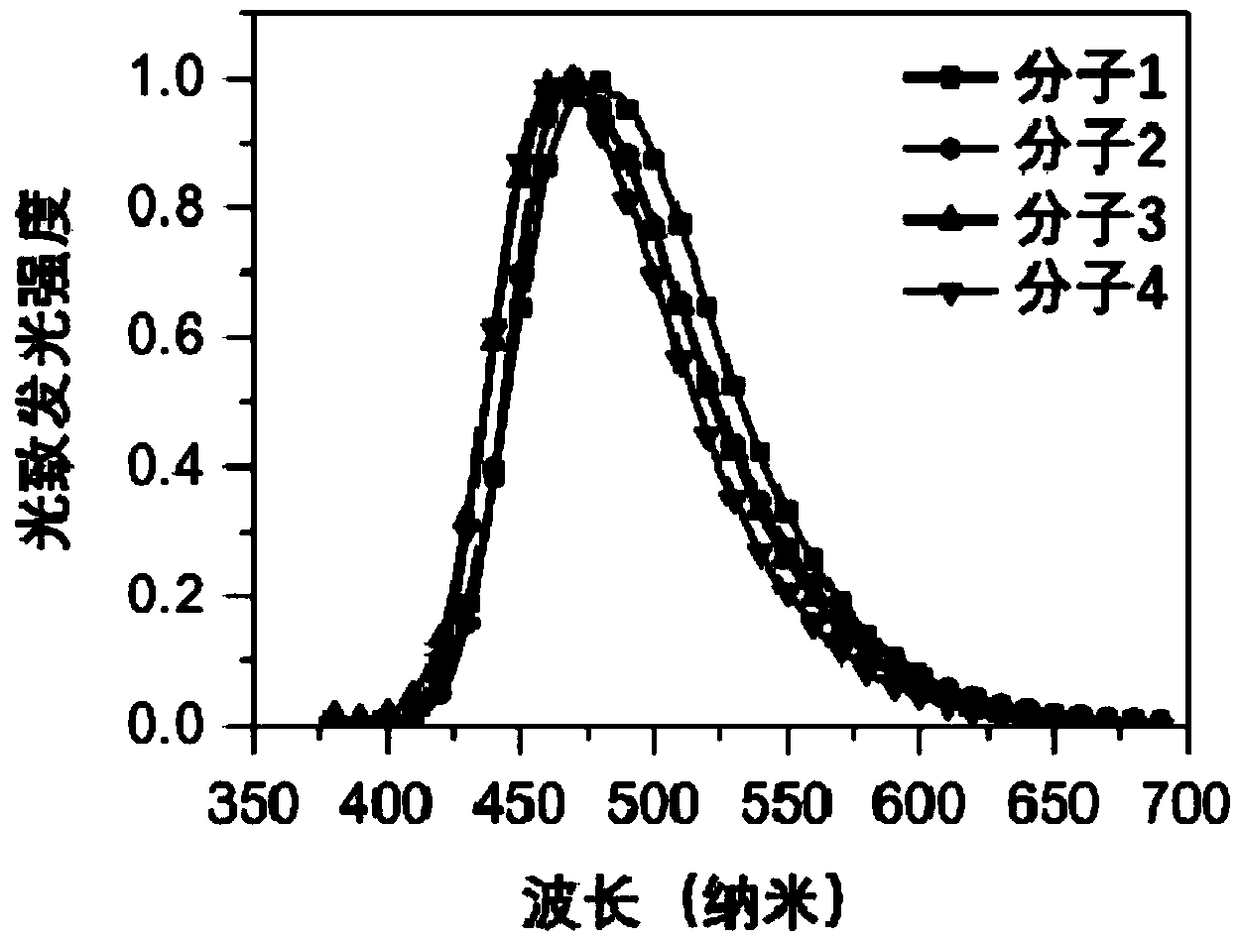
Organic small-molecule luminescent material and organic electroluminescent device
A technology of luminescent materials and small molecules, applied in luminescent materials, electrical solid devices, electrical components, etc., can solve the problems of limited organic small molecule optoelectronic materials, and achieve the effects of improving external quantum efficiency, single structure, and definite molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The synthetic method 1 of 2-bromo-N-phenylaniline, chemical reaction formula is as follows:
[0059]
[0060] In a 250mL three-necked flask, add aniline (0.2mol, 18.6g), o-bromoiodobenzene (0.2mol, 56.58g), palladium acetate (0.6mmol, 134.4mg), sodium tert-butoxide (0.4 mol) into the flask in turn, then add 150mL of toluene, pass N 2 20 minutes, then add tert-butylphosphine (1.2mmol, 1.2ml), continue to pass through N 2 20 minutes, heat to reflux and stir for 12 hours. The temperature was lowered to room temperature, sodium tert-butoxide was removed by suction filtration, the solvent was distilled off under pressure, and the solvent was separated and purified by silica gel chromatography to obtain a colorless oily liquid (27.8 g, yield 56%).
Embodiment 2
[0062] The synthetic method 2 of 2-bromo-N-phenylaniline, chemical reaction formula is as follows:
[0063]
[0064] In a 250mL three-necked flask, add aniline (0.2mol, 18.6g), o-bromoiodobenzene (0.2mol, 56.58g), palladium acetate (0.6mmol, 134.4mg), sodium tert-butoxide (0.4 mol) was added in the flask successively, o-bis(2-phenyl)bis(diphenylphosphine) (0.6mmol, 340mg), then added the toluene of 150mL, logical N 2 20 minutes, heat to reflux and stir for 12 hours. The temperature was lowered to room temperature, sodium tert-butoxide was removed by suction filtration, the solvent was distilled off under pressure, and the solvent was separated and purified by silica gel chromatography to obtain a colorless oily liquid (44.2 g, yield 89%).
[0065] After comparing the above Example 1 and Example 2, it can be found that by changing the catalyst ligand from t-butylphosphine to o-bis(2-phenyl)bis(diphenylphosphine), 2-bromo-N-benzene Aniline yield increased from 56% to 89%. ...
Embodiment 3
[0067] The preparation method 1 of (4-bromophenyl)-phenyl-carbamic acid tert-butyl ester, chemical reaction formula is as follows:
[0068]
[0069] In a 500 ml one-necked flask, di-tert-butyl dicarbonate (BOC) (0.2mol, 43.6g) was added to 400 ml of tetrahydrofuran at room temperature, and then p-N-(dibromophenyl)aniline (0.1mol , 24.8g), heated to reflux and stirred for 24 hours. The mixture was then poured into 1 L of water, and the product was extracted with dichloromethane. Dry the organic phase with anhydrous magnesium sulfate, remove the solvent after separation, and separate and purify with silica gel chromatography to obtain a colorless oily liquid (32.0 g, yield 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com