Folate receptor-mediated tumor internal environment-sensitive doxorubicin albumin nanoparticles and preparation method
A technology of albumin nanoparticles and folic acid receptors, which can be used in antitumor drugs, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. It can solve the problems of incomplete drug release and poor selectivity of nanoparticles. , to achieve the effect of achieving circulation stability, reducing drug toxicity and side effects, and increasing cellular uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
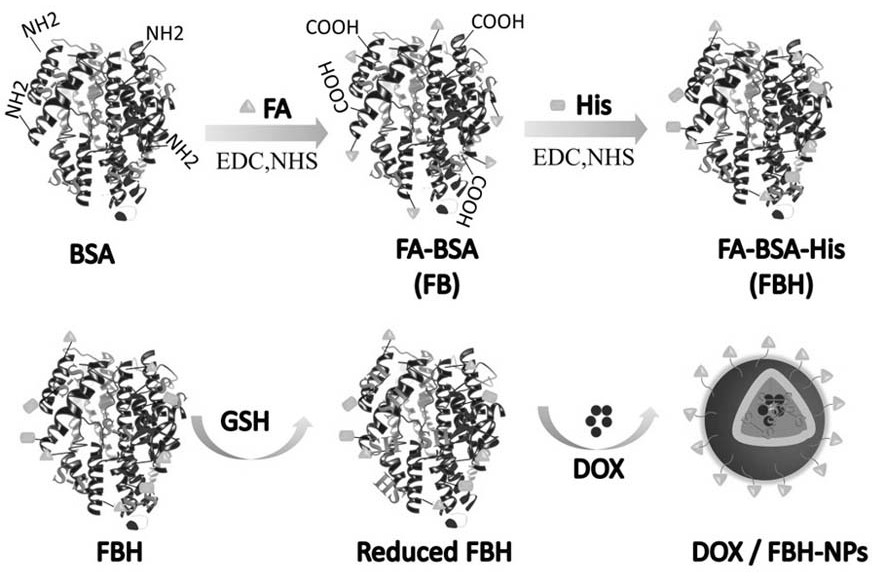
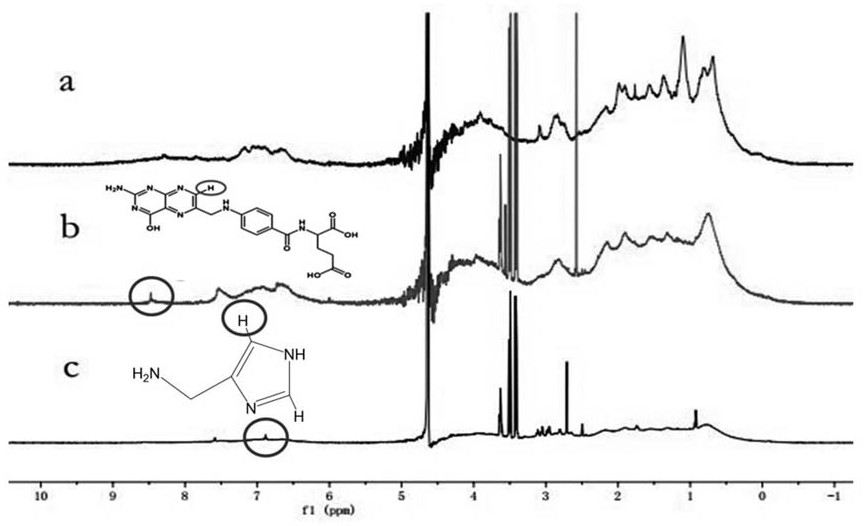
[0037] This embodiment provides a folate receptor-mediated pH and reduction dual-sensitive doxorubicin albumin nanoparticles. See attached figure 1 , is a schematic diagram of the preparation process of folic acid receptor-mediated pH and reduction double-sensitive doxorubicin albumin nanoparticles, through aminocarboxyl condensation reaction and one-pot amide reaction, folic acid is grafted on the surface of bovine serum albumin successively and histamine, and then open the disulfide bond of the folic acid-albumin-histamine modifier through GSH, mix with the doxorubicin solution, and finally the disulfide bond spontaneously cross-links to form nanoparticles.
[0038] The specific preparation method of the present embodiment comprises the following steps:
[0039] 1. Synthesis of folic acid-albumin modifier:
[0040] Dissolve 88 mg folate acid (FA) in 4 mL dimethyl sulfoxide (DMSO), sonicate for 10 min, and add 58 mg 1-(3-dimethylaminopropyl)-3-ethyl carbon under stirring D...
Embodiment 2
[0050] This example investigates the particle size change of DOX / FBH-NPs prepared in Example 1 under the conditions of different pH and GSH concentration.
[0051] Filter the DOX / FBH-NPs with a 0.22 μM filter membrane, and sonicate for 5-10 minutes. The DOX / FBH-NPs solution was incubated in the following environments: phosphate buffered saline (PBS) at pH 7.4, PBS at pH 5.3, PBS at pH 7.4 and 10mM GSH, and the volume ratio of PBS to nanoparticle solution was 5: 1. The incubation time is 2 hours, the incubation temperature is 37°C, and then placed in a cuvette, put into the sample pool, and the particle size change is measured by a Malvern particle size analyzer. For the results, see the attached Image 6 ,Depend on Image 6 It can be seen that the particle size of DOX / FBH-NPs increases in PBS at pH 5.3, but the disulfide bonds of nanoparticles are opened under the condition of high concentration of GSH, and the particle size is disordered. This result proves that DOX / FBH-NPs...
Embodiment 3
[0053] In this example, the drug-loading behavior of DOX / FBH-NPs prepared in Example 1 was investigated.
[0054] Establish DOX standard curve equation 1: Accurately weigh an appropriate amount of DOX, use water as solvent, and dilute to the following concentrations: 100 μg / mL, 80 μg / mL, 40 μg / mL, 20 μg / mL, 10 μg / mL, 5 μg / mL, 2.5 μg / mL, 1.25 μg / mL, 0.625 μg / mL. The absorbance was measured with a multifunctional microplate reader. Do a linear regression with the DOX concentration on the absorbance value to obtain the standard curve equation: y=240.46x-11.213 (r 2 =0.9991).
[0055] The drug loading and encapsulation efficiency of DOX / FBH-NPs were calculated by formula (1) and formula (2), respectively.
[0056] The formula (1) for calculating the drug loading of DOX / FBH-NPs: the amount of drug loaded / the weight of the carrier; The difference in the amount of DOX in the serum.
[0057] The encapsulation efficiency calculation formula (2) of DOX / FBH-NPs: entrapped drug amoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com