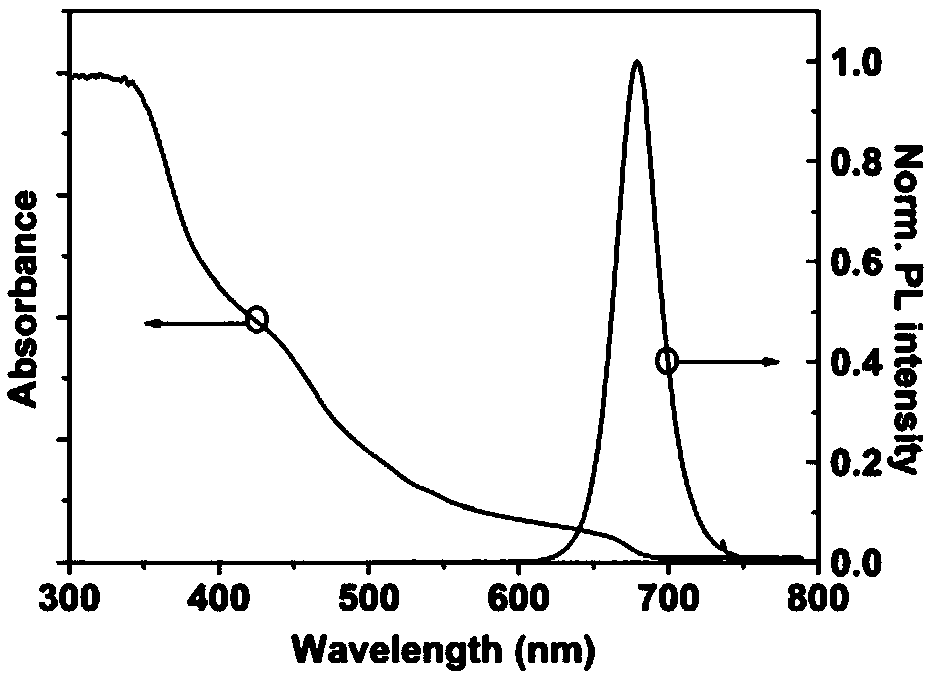
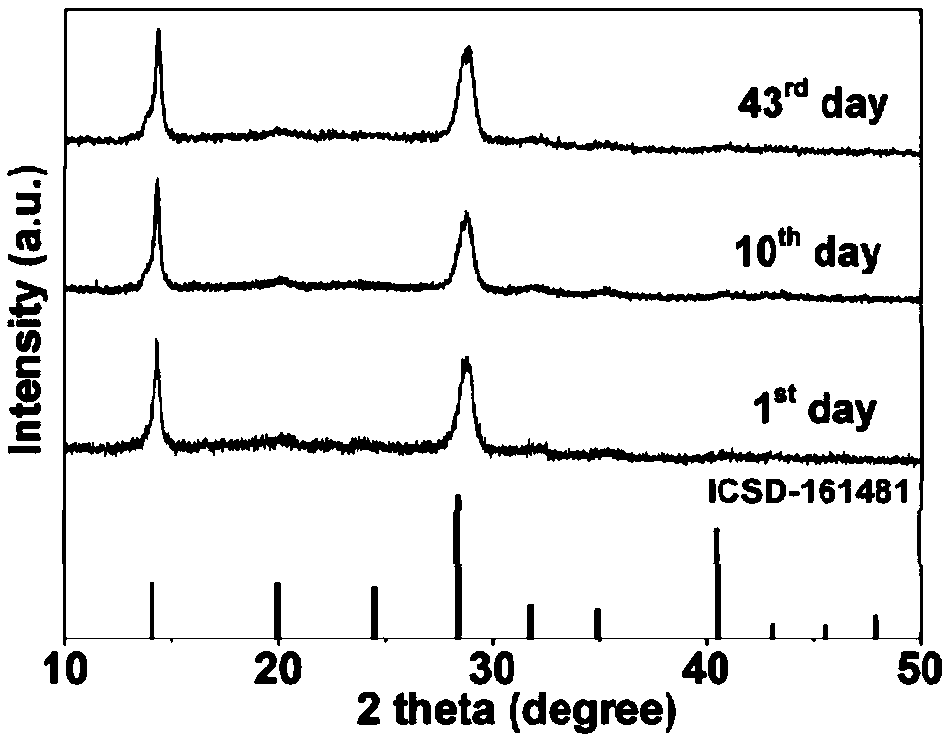
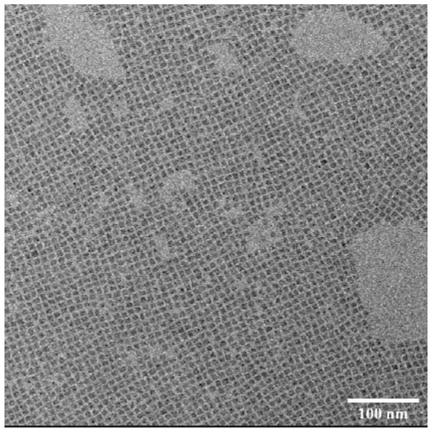
Synthesizing method of iodine-lead perovskite quantum dot
A technology of lead-iodine perovskite and synthesis method, applied in chemical instruments and methods, preparation of amino compounds from amines, nanotechnology, etc., to achieve high fluorescence quantum efficiency, simple operation, and easy large-scale application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Will Cs 2 CO 3 (analytical pure) and oleic acid (analytical pure) were mixed according to the stoichiometric ratio of 1:3, then 5 mL of octadecene (analytical pure) was added, nitrogen gas was introduced, and the mixture was heated to 120 ° C until the powder was completely dissolved and then cooled to room temperature. The concentration of Cs ions in the resulting solution was 5.0 mol / 42.5 mL. Mix PbO (analytical pure) and oleic acid in a stoichiometric ratio of 1:3, then add 5 mL of octadecene, blow in nitrogen, heat the mixture to 120 °C, and cool to room temperature until the powder is completely dissolved. The concentration of Pb ions in the obtained solution was 1.0 mol / L. After the above two solutions were preheated at 80°C, 0.4mL and 0.5mL were mixed with 0.4mL oleylamine and 5mL octadecene respectively, and the mixture was heated to 150°C, followed by rapid injection of 0.2mL iodotrimethylsilane, and the reaction After 5s, cool the device with an ice-water b...
Embodiment 2
[0024] Will Cs 2 CO 3 (analytical pure) and oleic acid (analytical pure) were mixed according to the stoichiometric ratio of 1:3, then 5 mL of octadecene (analytical pure) was added, nitrogen gas was introduced, and the mixture was heated to 120 ° C until the powder was completely dissolved and then cooled to room temperature. The concentration of Cs ions in the resulting solution was 5.0 mol / 42.5 mL. Mix PbO (analytical pure) and oleic acid in a stoichiometric ratio of 1:3, then add 5 mL of octadecene, blow in nitrogen, heat the mixture to 120 °C, and cool to room temperature until the powder is completely dissolved. The concentration of Pb ions in the obtained solution was 1.0 mol / L. After preheating the above two solutions at 80°C, mix 0.4mL and 0.5mL with 0.5mL oleylamine and 5mL octadecene respectively, heat the mixture to 150°C, then inject 0.2mL iodotrimethylsilane rapidly, and react After 5s, cool the device with an ice-water bath, and the resulting solution is CsPb...
Embodiment 3
[0026] Will Cs 2 CO 3 (analytical pure) and oleic acid (analytical pure) were mixed according to the stoichiometric ratio of 1:2, then 5 mL of octadecene (analytical pure) was added, nitrogen gas was introduced, and the mixture was heated to 120 °C until the powder was completely dissolved and then cooled to room temperature. The concentration of Cs ions in the resulting solution was 5.0 mol / 42.5 mL. Mix PbO (analytical pure) and oleic acid in a stoichiometric ratio of 1:2, then add 5 mL of octadecene, blow in nitrogen, heat the mixture to 120 °C, and cool to room temperature until the powder is completely dissolved. The concentration of Pb ions in the obtained solution was 1.0 mol / L. After the above two solutions were preheated at 80°C, 0.4mL and 0.5mL were mixed with 0.4mL oleylamine and 5mL octadecene respectively, and the mixture was heated to 150°C, followed by rapid injection of 0.2mL iodotrimethylsilane, and the reaction After 5s, cool the device with an ice-water ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com