A HPLC method for the simultaneous determination of eight active components in Codonopsis pilosula
A technology for active ingredients and ginseng medicinal materials, applied in the field of quality control of traditional Chinese medicinal materials and their preparations, can solve problems such as insufficient description of the quality of Codonopsis pilosula medicinal materials, and achieve the effects of good linear relationship, simple operation and rapid separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
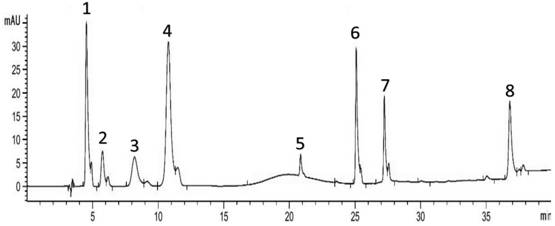
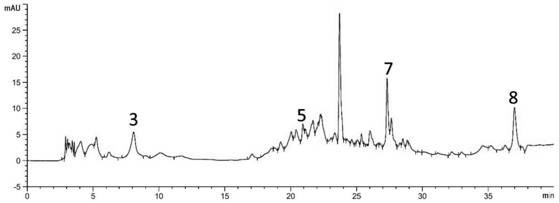
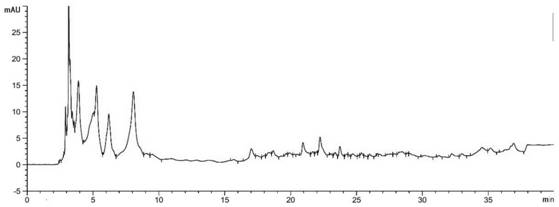
[0019] Standard solution preparation: Accurately weigh 10mg, 9.9mg, 10mg, 10.3 mg, 40mg, 10.7mg, 9.5mg, 10.1mg, add an appropriate amount of methanol to dissolve, respectively dilute to the mark in a 10mL volumetric flask, and shake well to obtain tangshenoside, nicotinic acid, adenosine, syringin, succinic acid, a The concentrations of fulmic acid, 5-hydroxymethylfurfural and atractylodele III are 1mg / mL, 0.99mg / mL, 1mg / mL, 0.103mg / mL, 4mg / mL, 0.107mg / mL, 0.95mg / mL, 0.101 mg / mL. Store them separately as standard stock solution for future use.
[0020] Preparation of sample solution: Accurately weigh 1 g of dried Codonopsis pilosula, place in a 150 mL Erlenmeyer flask, add 25 mL of methanol, ultrasonicate for 15 min, take the supernatant and filter it with a 0.45 μm filter membrane, and store it for later use.
[0021] Establishment of standard curves for the determination of active components in eight Codonopsis medicinal materials: the chromatographic conditions are C18 ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com