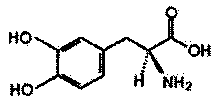
Levodopa refining method
A technology of levodopa and phenol, which is applied in the field of medicine, can solve the problems of lack of levodopa purity and interaction effects, and achieve the effects of strong selectivity, improved quality, and high purity of fine products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for refining levodopa, the steps are:
[0027] (1) Preparation of extraction solution: Take phenol and water, mix them according to the weight ratio of 1:3, heat to 70°C to make them miscible, and obtain the extraction solution;
[0028] (2) Dissolution: Take the crude product of levodopa, add 3 times the weight of the extract, dissolve it to saturation at 70°C, let it stand for 40 minutes, and then filter it at 65°C;
[0029] (3) Impurity removal: take the filtrate to cool, precipitate levodopa and phenol, and filter the precipitate;
[0030] (4) Phenol removal: take the precipitate obtained in step (3), add absolute ethanol 5 times its weight, dissolve the phenol and remove it to obtain levodopa solids;
[0031] (5) Recrystallization: Take the solid levodopa, add 8 times its weight of hydrochloric acid with a mass fraction of 0.05 mol / L, adjust the pH to 1.5, heat to boiling, add the solid levodopa to dissolve to saturation, put Cool to make levodopa crysta...
Embodiment 2
[0033] A method for refining levodopa, the steps are:
[0034] (1) Preparation of extraction solution: Take phenol and water, mix them according to the weight ratio of 1:4, heat to 68°C to make them miscible, and obtain the extraction solution;
[0035] (2) Dissolution: Take the crude product of levodopa, add 5 times its weight in the extract, dissolve it to saturation at 68°C, let it stand for 50 minutes, and then filter it at 68°C;
[0036] (3) Impurity removal: take the filtrate to cool, precipitate levodopa and phenol, and filter the precipitate;
[0037] (4) Phenol removal: Take the precipitate obtained in step (3), add 7 times its weight in absolute ethanol, dissolve the phenol and remove it to obtain levodopa solids, and repeat this operation again;
[0038] (5) Recrystallization: Take the solid levodopa, add 6 times its weight of hydrochloric acid with a mass fraction of 0.1 mol / L, adjust the pH to 1.2, heat to boiling, add the solid levodopa to dissolve to saturation, ...
Embodiment 3
[0040] A method for refining levodopa, the steps are:
[0041] (1) Preparation of extraction solution: Take phenol and water, mix them according to the weight ratio of 1:5, heat to 65°C to make them miscible, and obtain the extraction solution;
[0042] (2) Dissolution: Take the crude product of levodopa, add 4 times its weight in the extract, dissolve it to saturation at 65°C, let it stand for 60 minutes, and then filter it at 70°C;
[0043] (3) Impurity removal: take the filtrate to cool, precipitate levodopa and phenol, and filter the precipitate;
[0044] (4) Phenol removal: Take the precipitate obtained in step (3), add absolute ethanol 8 times its weight, dissolve the phenol and remove it to obtain levodopa solids, repeat this operation twice;
[0045] (5) Recrystallization: Take the solid levodopa, add 6 times its weight of hydrochloric acid with a mass fraction of 0.12 mol / L, adjust the pH to 1.0, heat to boiling, add the solid levodopa to dissolve to saturation, put ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com