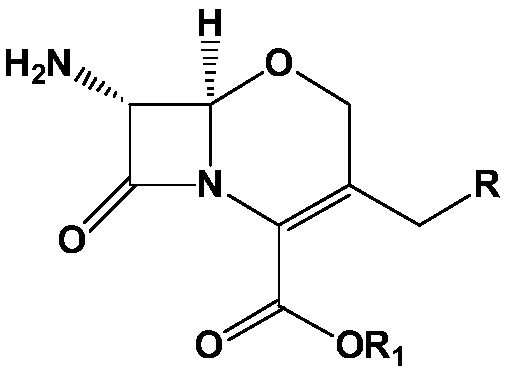
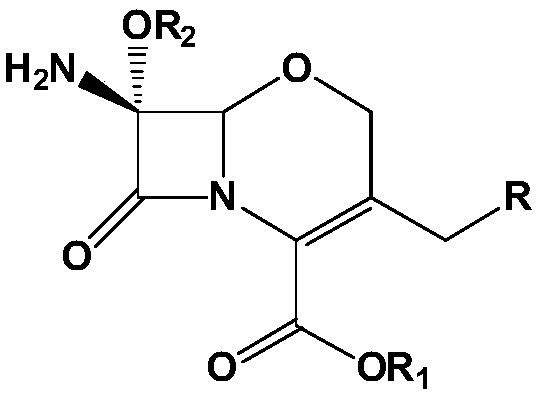
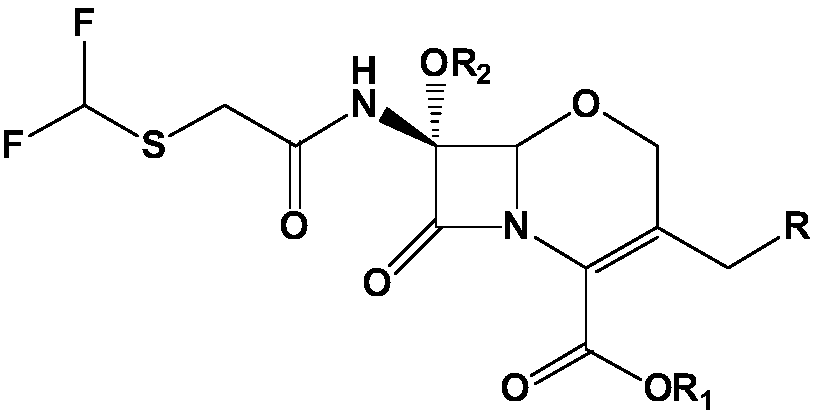
METHOD FOR MANUFACTURING 7alpha-ALKOXYOXACEPHEM INTERMEDIATE COMPOUND
A technology for alkoxy cephem and intermediates, which is applied in the field of preparation of 7α-alkoxy cephem intermediates, can solve problems such as unfavorable, expensive photoreaction equipment, low yield and the like, and achieves easy mass production , excellent yield, simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1. (6R,7R)-diphenylmethyl-7-(difluoromethylthio)acetamido)-3-(chloromethyl)-7-methoxy-8-oxo-5 Preparation of oxa-1-aza-bicyclo[4,2,0]oct-2-ene-2-carboxylate
[0085] As starting material, 50.0 g (0.13 mol) of 7-amino-3-chloromethyl-1-oxa-3-cephem-4-carboxylic acid diphenyl ester were injected with 450 g of dichloromethane, and After cooling to -20°C, 29.0 g (0.4 moles) of chlorine was injected for 60 minutes. After stirring for 10 minutes at the same temperature, 174.7 g (0.46 mol) of a methanol solution of 10% LiOMe (lithium methoxy) was dropped at a temperature of -40°C to -50°C. Then, after stirring for 5 minutes at the same temperature, 6.4 g (0.11 mol) of acetic acid was added. 428.5 g of 10% sodium sulfite and 400 g of purified water were sequentially added to the above reaction solution. After stirring for 30 minutes, the dichloromethane layer was separated and washed with dilute sodium bicarbonate. After adding 50 g of anhydrous magnesium sulfate to ...
Embodiment 2
[0089] Example 2. (6R,7R)-diphenylmethyl-7-amino-7-methoxy-3-((1-methyl-1H-tetrazol-5-ylthio)methyl)-8 - Preparation of oxo-5-oxa-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate
[0090] As starting material, 50.0 g (0.13 mol) of 7-amino-3-chloromethyl-1-oxa-3-cephem-4-carboxylic acid diphenyl ester were injected with 450 g of dichloromethane, and After cooling to -20°C, 29.0 g (0.4 moles) of chlorine was injected for 60 minutes. After stirring for 10 minutes at the same temperature, 174.7 g (0.46 mol) of a methanol solution of 10% LiOMe (lithium methoxy) was dropped at a temperature of -40°C to -50°C. Then, after stirring for 5 minutes at the same temperature, 6.4 g (0.11 mol) of acetic acid was added. 428.5 g of 10% sodium sulfite and 400 g of purified water were sequentially added to the above reaction solution. After stirring for 30 minutes, the dichloromethane layer was separated and washed with dilute sodium bicarbonate. After adding 50 g of anhydrous magnesium sulfate t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com