Tumor homing cell-penetrating peptide tLyP-1 modified apoferritin nano-cage and preparation method thereof
A technology of apoferritin and tlyp-1, applied in the field of biomedicine, can solve the problem of single targeting, achieve the effect of reducing dosage and improving therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of the apoferritin nanocage modified by the tumor-homing penetrating peptide tLyP-1 of the present invention comprises the following steps:
[0034] (1) Construction of recombinant protein cage expression engineering bacteria
[0035] Based on the HFtn coding gene, modify the coding gene TGTGGTAATAAACGTACCCGTGGTGGTGGTGGTAGC at the 5' end of the tumor-homing membrane-penetrating peptide tLyP-1 and the connecting peptide GGGGS, and subclone the gene sequence into the pET-20b(+) plasmid vector to obtain A plasmid containing the gene of interest. The plasmid was heat-shocked into Escherichia coli competent cells, and positive single clones were selected by means of ampicillin resistance and gene sequencing, etc., and the positive single clones were cultured in large quantities, and the recombinant bacteria were preserved in 10% glycerol. (2) Expression of recombinant protein and collection of strains
[0036] Inoculate the engineered bacteria expres...
Embodiment 1
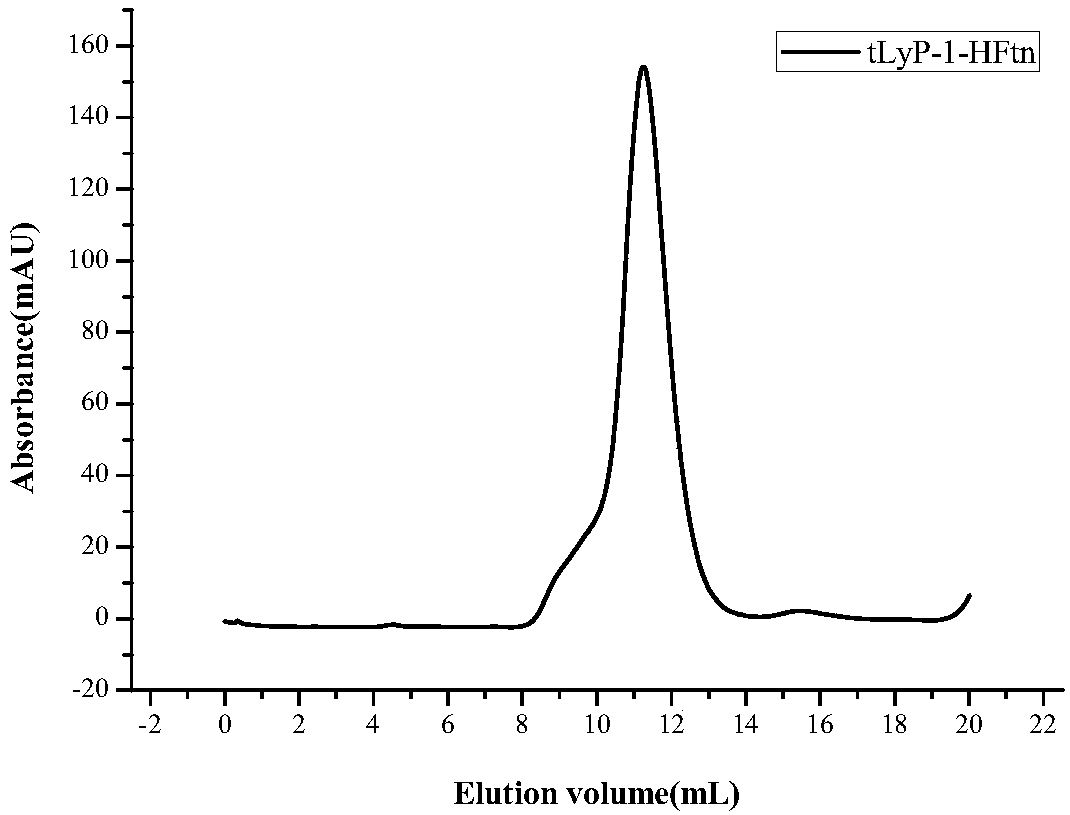
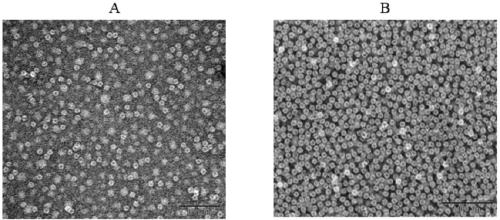
[0041] Such as figure 1 As shown in Figure 4, the construction of pET-20b(+) / tLyP-1-HFtn expressing recombinant engineering bacteria
[0042] Add the TGTGGTAATAAACGTACCCGTGGTGGTGGTGGTAGC sequence at the 5' end of the HFtn gene coding sequence reported in the literature,
Embodiment 2
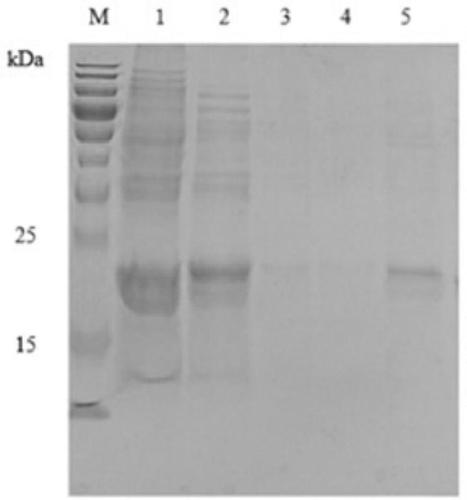
[0044] Analysis of the soluble expression form of tLyP-1-HFtn target recombinant protein
[0045] Add the positive recombinant bacteria to the LB medium containing ampicillin, shake at 37°C and 200r / min overnight to activate the strain. Transfer 1% of the inoculum to 5 mL of LB medium containing ampicillin, culture at 37°C and 210 r / min until the OD600 of the bacterial solution reaches 0.6-0.8, add IPTG with a final concentration of 0.5mM to the medium, and induce at 30°C Cultivate for 6h. Take 2mL of bacterial liquid, centrifuge at 4°C, 8000×g for 5min to collect the bacterial cells, discard the supernatant, add 200μL of binding buffer (50mM NaH2PO4, 300mM NaCl, 10Mm imidazole, pH=7.9) to resuspend, under the same conditions Centrifuge again, discard the supernatant to collect the bacteria, resuspend in 200 μL of binding buffer again, and store at 4°C. The bacteria were ultrasonically disrupted, and the sample was taken out as the total bacteria; the supernatant was collect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com