Drug-related genotyping kit and typing method for thiopurine drug
A genotyping and kit technology, applied in the direction of biochemical equipment and methods, microbial measurement/inspection, etc., to avoid adverse drug reactions, improve clinical treatment effect, and increase concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
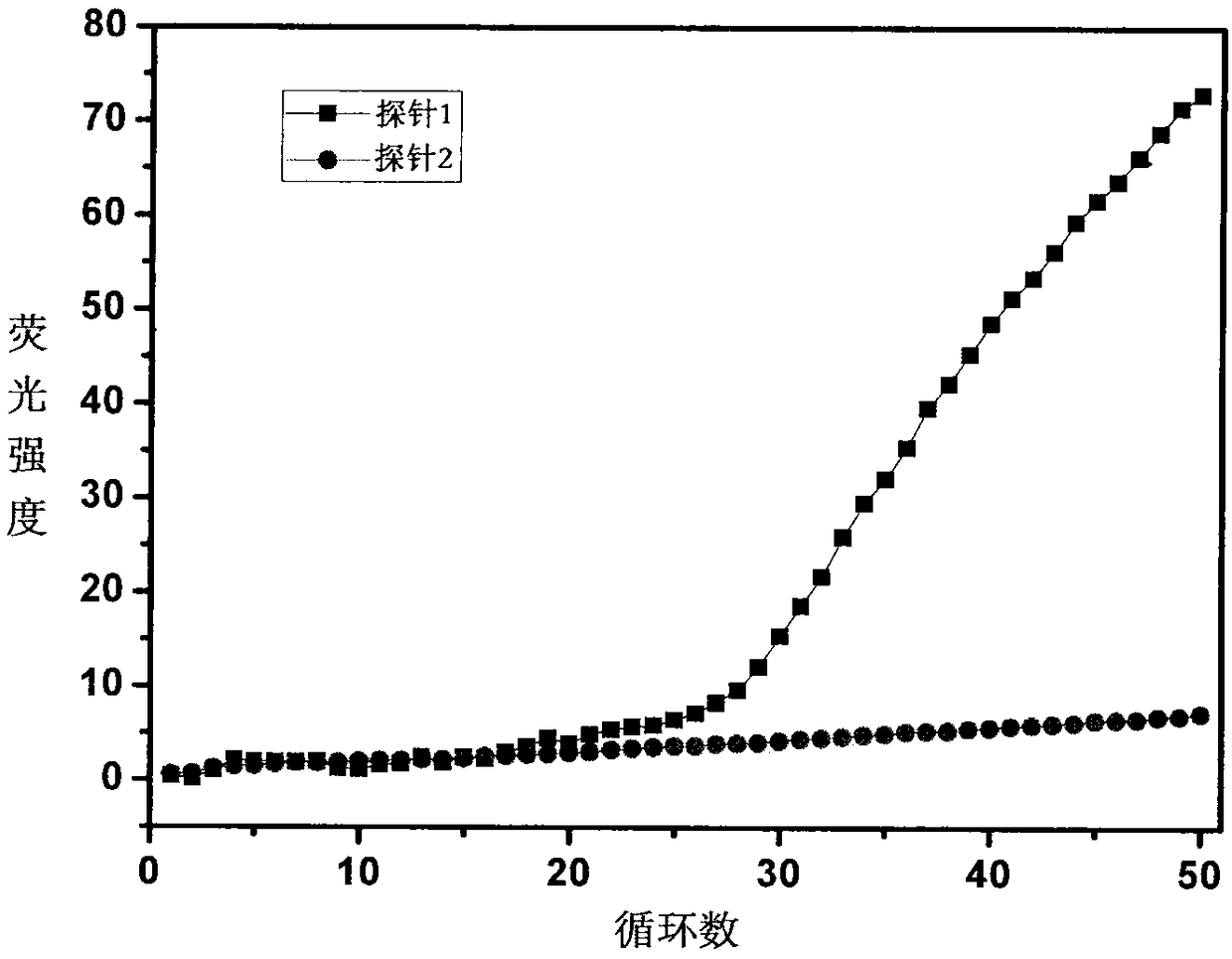
Image
Examples
experiment example 1
[0069] This embodiment provides a nucleic acid amplification reagent, including the following components:
[0070] Taq enzyme, 300mg / mL;
[0071] dNTPs, 100g / L;
[0072] Tris-HCl, 1mL / L;
[0073] MgCl 2 , 20g / L;
[0074] KCl, 10g / L;
[0075] (NH 4 ) 2 SO 4 , 10g / L;
[0076] Nano silica, 50g / L;
[0077] Triton X-100, 2mL / L.
[0078] The preparation method of nano-silica comprises the following steps:
[0079] (1) The solution I and the solution II are stirred and preheated to a reaction temperature of 110° C. on a heating stirrer, mixed and added to a three-necked flask of a thermal constant temperature heating magnetic stirrer. Wherein, solution I is obtained by mixing ammonia water: water: ethanol in a volume ratio of 20: 1: 79, and ammonia water is an aqueous solution with an ammonia content of 25% by weight; solution II is mixed by ethyl orthosilicate and ethanol in a volume ratio of 1: 4 get;
[0080] (2) stirring for 5 minutes, the mixed solution of solution ...
experiment example 2
[0085] This embodiment provides a nucleic acid amplification reagent, including the following components:
[0086] Taq enzyme, 500mg / mL;
[0087] dNTPs, 50g / L;
[0088] Tris-HCl, 0.5mL / L;
[0089] MgCl 2 , 10g / L;
[0090] KCl, 5g / L;
[0091] (NH 4 ) 2 SO 4 , 5g / L;
[0092] Nano silica, 100g / L;
[0093]Triton X-100, 5mL / L.
[0094] The preparation method of nano-silica comprises the following steps:
[0095] (1) The solution I and the solution II are stirred and preheated to a reaction temperature of 100° C. on a heating stirrer, mixed and added to a three-necked flask of a thermal constant temperature heating magnetic stirrer. Wherein, solution I is obtained by mixing ammonia water: water: ethanol in a volume ratio of 5: 1: 44, and ammonia water is an aqueous solution with an ammonia content of 26 wt %; solution II is mixed by ethyl orthosilicate and ethanol in a volume ratio of 3: 17 get;
[0096] (2) stirring for 5 minutes, the mixed solution of solution I and s...
experiment example 3
[0099] This embodiment provides a nucleic acid amplification reagent, including the following components:
[0100] Taq enzyme, 100mg / mL;
[0101] dNTPs, 200g / L;
[0102] Tris-HCl, 2mL / L;
[0103] MgCl 2 , 40g / L;
[0104] KCl, 20g / L;
[0105] (NH 4 ) 2 SO 4 , 40g / L;
[0106] Nano silica, 20g / L;
[0107] Triton X-100, 1mL / L.
[0108] The preparation method of nano-silica comprises the following steps:
[0109] (1) The solution I and the solution II are stirred and preheated to a reaction temperature of 120° C. on a heating stirrer, mixed and added to a three-necked flask of a thermal constant temperature heating magnetic stirrer. Wherein, solution I is obtained by mixing ammonia water: water: ethanol in a volume ratio of 60: 1: 139, and ammonia water is an aqueous solution with an ammonia content of 27wt%; solution II is mixed by ethyl orthosilicate and ethanol in a volume ratio of 3:7 get;
[0110] (2) stirring for 5 minutes, the mixed solution of solution I and so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com