A kind of preparation method of Aniracetam
An Aniracetam and synthetic route technology, applied in the field of Aniracetam preparation, can solve the problems of difficult separation and purification of products, harsh reaction conditions, long synthesis routes, etc., and achieves cheap and easy-to-obtain raw materials, short reaction steps, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
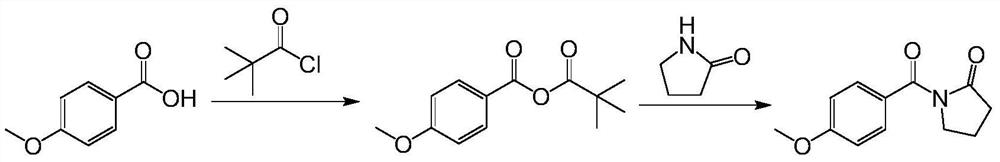
[0034] According to the preferred embodiment provided by the present invention, the preparation method of Aniracetam comprises the following specific steps:
[0035] S1: Add the organic solvent, p-methoxybenzoic acid, and organic base into the flask together, then slowly add pivaloyl chloride dropwise under the cooling condition of an ice-water bath, after the drop is completed, stir the reaction for 0.5 to 8 hours,
[0036] S2: Add 2-pyrrolidone into the flask, heat to reflux, continue to stir and react for 6 to 24 hours, the reaction is complete, wash with water, wash with alkali, dry, concentrate, dissolve with ethanol, decolorize with activated carbon, filter while hot, cool and crystallize, Filter again, take the filter cake, and dry under reduced pressure to obtain the target product Aniracetam.
[0037] In a preferred embodiment, the organic base is selected from any one or more of the following: triethylamine, diisopropylethylamine, pyridine, N-methylmorpholine, tetram...
Embodiment 1
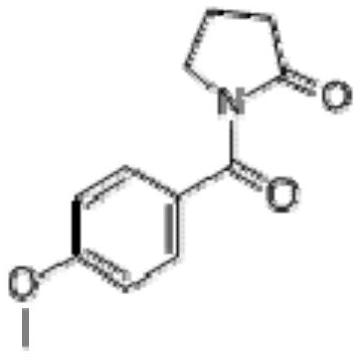
[0043] Add dichloromethane (250ml) in the flask, p-methoxybenzoic acid (30.4g), triethylamine (60.7g), slowly add pivaloyl chloride (25.3g) dropwise under ice-water bath cooling condition, dropwise finishes, Heat to reflux, stir for 2 hours; then, add 2-pyrrolidone (25.5 g), heat to reflux, continue to stir and react for 6 to 24 hours, until the reaction is complete, wash with water, wash with sodium carbonate aqueous solution, dry, concentrate, dissolve with ethanol, Activated carbon was decolorized, filtered while it was hot, cooled and crystallized, filtered again, the filter cake was taken, and dried under reduced pressure to obtain the target product Aniracetam (30.7g), with a yield of 70.2%.
[0044] Wherein, Aniracetam is characterized as follows:
[0045] 1 H NMR (400MHz, DMSO, TMS): δ7.57 (2H, d, J = 8.8Hz), 6.94 (2H, d, J = 8.8Hz), 3.81 (3H, s), 3.79~3.75 (2H, m ), 2.54~2.43(2H,m), 2.04~1.99(2H,m).
Embodiment 2
[0047] Add dichloromethane (250ml) in the flask, p-methoxybenzoic acid (30.4g), diisopropylethylamine (77.4g), slowly add pivaloyl chloride (25.3g) dropwise under ice-water bath cooling condition, After dripping, heat to reflux, stir and react for 2 hours; then, add 2-pyrrolidone (25.5 g), heat to reflux, continue to stir and react for 6 to 24 hours, until the reaction is complete, wash with water, wash with sodium hydroxide aqueous solution, dry, and concentrate, Dissolve with ethanol, decolorize with active carbon, filter while hot, cool and crystallize, filter again, take filter cake, dry under reduced pressure, obtain target product Aniracetam (31.2g), yield 71.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com