Bicyclo-orange flower naphthene type sesquiterpene derivative as well as preparation and application thereof
A technology of nerane and sesquiterpene is applied in the field of bicyclic cyclonerane-type sesquiterpene derivatives and their preparation, and can solve the problems of secondary environmental pollution, drug residues, non-red tide biological hazards and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
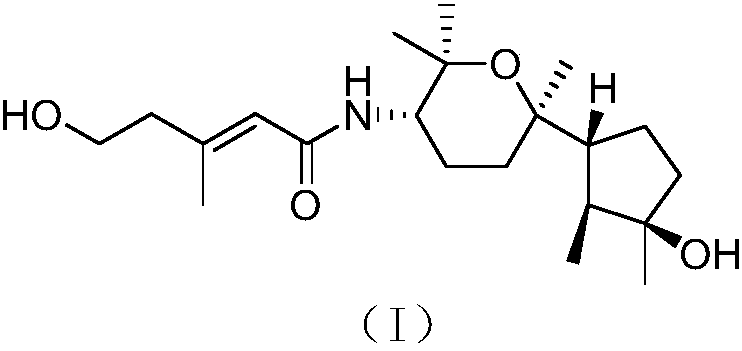
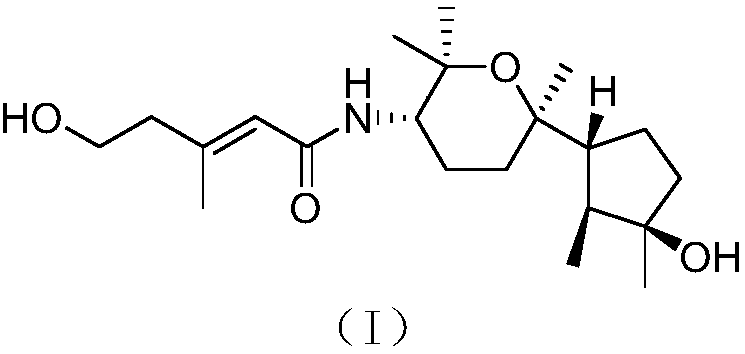
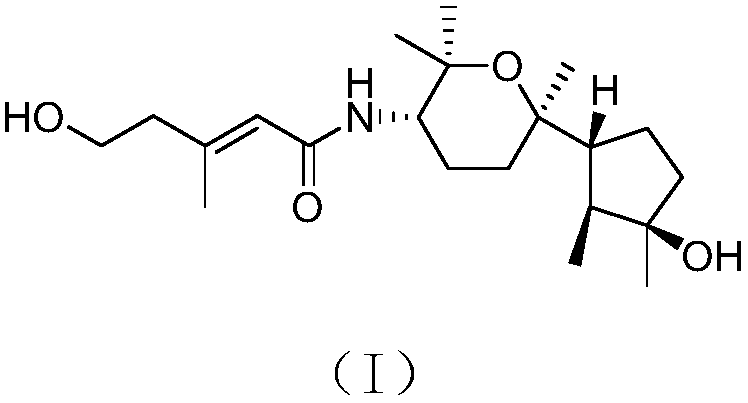
[0024] The structure of the bicyclic nerolitan-type sesquiterpene derivative derived from seaweed endophytic fungi is shown in formula (I).
[0025]
[0026] The compound has the following physicochemical and spectroscopic properties:
[0027] Colorless oil; specific rotation [α] 20 D -30(c 0.32, MeOH); H NMR spectrum (solvent is deuterated chloroform) δ H 1.02d(6.6),1.71m,1.61m,1.50m,1.80m,1.50m,1.75m,1.70m,1.45ddd(12.1,3.3,3.3),1.67m,3.87ddd(11.5,9.9,4.1), 1.16s, 1.24s, 1.19s, 1.19s, 5.62br s, 2.35br t(5.7), 3.79br t(5.7), 2.16br s, 5.24br d(9.8); C NMR spectrum (solvent is deuterated Chloroform) δ C 15.1 (CH 3 ), 44.0(CH), 81.6(C), 40.6(CH 2 ), 25.1 (CH 2 ), 56.6(CH), 75.8(C), 31.7(CH 2 ),23.6(CH 2 ), 53.3(CH), 73.9(C), 30.3(CH 3 ),26.2(CH 3 ),25.5(CH 3 ),22.9(CH 3 ), 166.3(C), 120.7(CH), 150.4(C), 43.7(CH 2 ),60.2(CH 2 ), 18.3 (CH 3 ); high-resolution mass spectrometry [M] + m / z 367.2725, calculated 367.2723.
Embodiment 2
[0029] The preparation method of the bicyclic ring nerolitan type sesquiterpene derivatives as shown in formula (I):
[0030] Get the Trichoderma asperellum (Trichoderma asperellum) A-YMD-9-2 strain that grows well on the plate, cut into small pieces and inoculate in rice solid medium, put 50 grams of rice solid medium in every 1 liter of Erlenmeyer flask , 200 bottles in total, fermented statically at room temperature for 40 days, then extracted three times with ethyl acetate, concentrated under reduced pressure, and obtained 212.4 grams of crude extract after concentration.
[0031] The rice solid medium consists of 500 grams of rice per liter, 6 grams of peptone, 500 milliliters of distilled water, and 500 milliliters of aged seawater.
[0032] Trichoderma asperellum (Trichoderma asperellum) A-YMD-9-2 strain was preserved in the China Center for Type Culture Collection CCTCC on June 27, 2018, address: Wuhan University, China, the preservation number is CCTCC M 2018405, and ...
Embodiment 3
[0037] The difference from Example 2 is that
[0038] Get the well-grown Trichoderma asperellum (Trichoderma asperellum) A-YMD-9-2 bacterial classification on the plate, cut into small pieces and inoculate in Jerusalem artichoke glucose liquid medium, put 300 milliliters of medium in every liter of Erlenmeyer flask, A total of 200 bottles were fermented at room temperature for 40 days, then extracted three times with dichloromethane, concentrated under reduced pressure, and 200 grams of crude extract was obtained after concentration.
[0039] The Jerusalem artichoke glucose liquid medium is composed of 500 milliliters of boiled juice containing 200 grams of Jerusalem artichoke tubers per liter, 20 grams of glucose, 5 grams of peptone, and 500 milliliters of aged seawater.
[0040]The crude extract was subjected to 200-300 mesh silica gel column chromatography, followed by volume ratios of 50:1, 30:1, 15:1, 10:1, 5:1, 2:1, 1:1 to 0:1 Petroleum ether-ethanol for gradient elutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com