Low-immunogenic bone defect part filling material and preparation method thereof
A technology for filling materials and bone defects, applied in medical science, prostheses, tissue regeneration, etc., can solve the problems of safety hazards, strong immunogenicity, etc., and achieve the goal of preventing fiber formation, reducing immunogenicity, and ensuring complete recovery Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
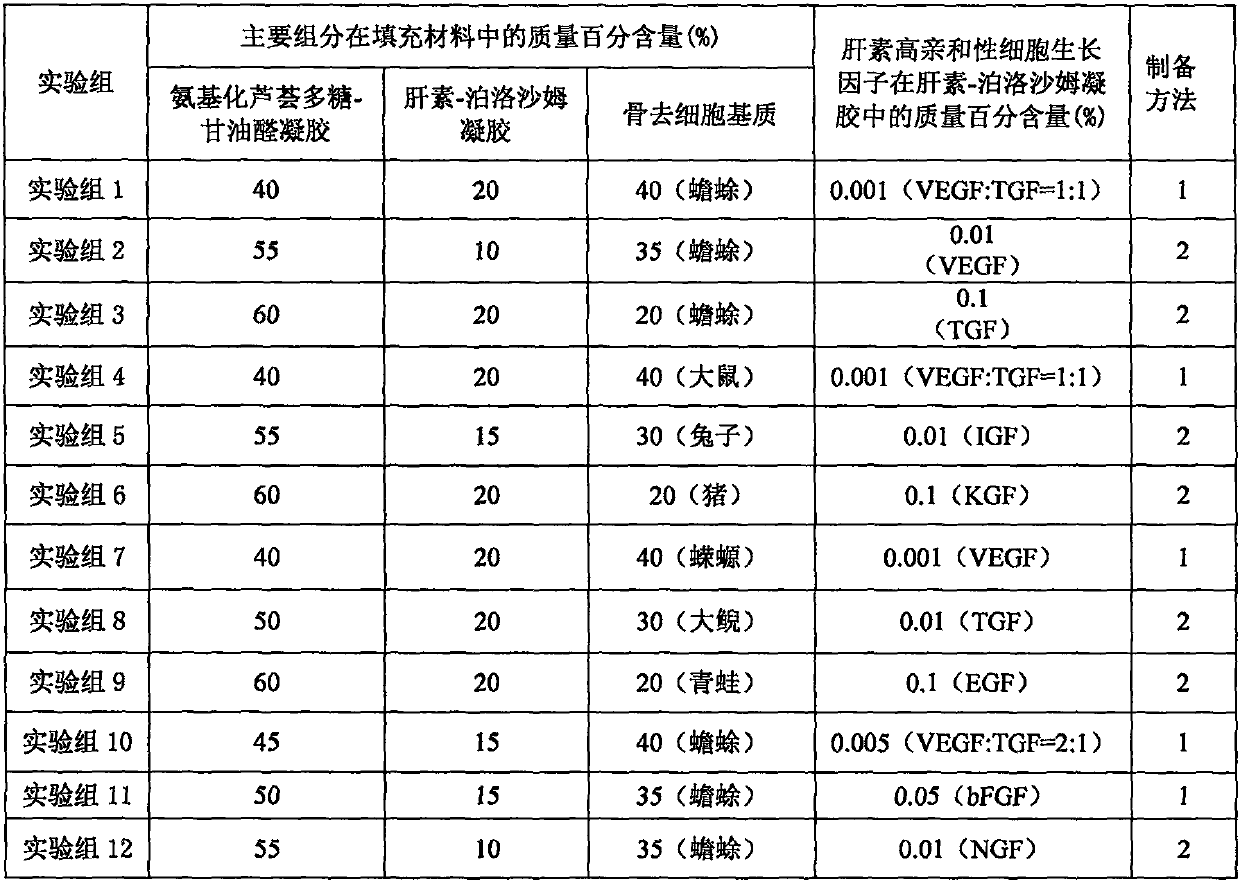
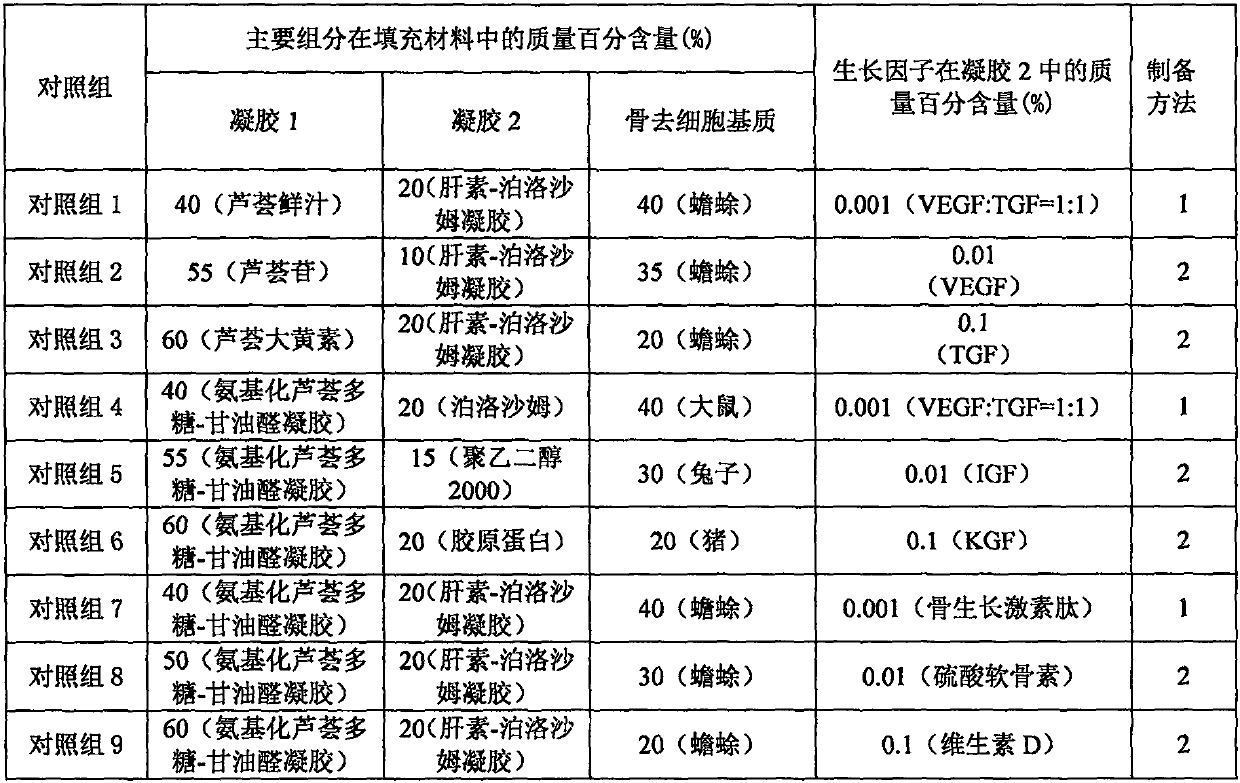
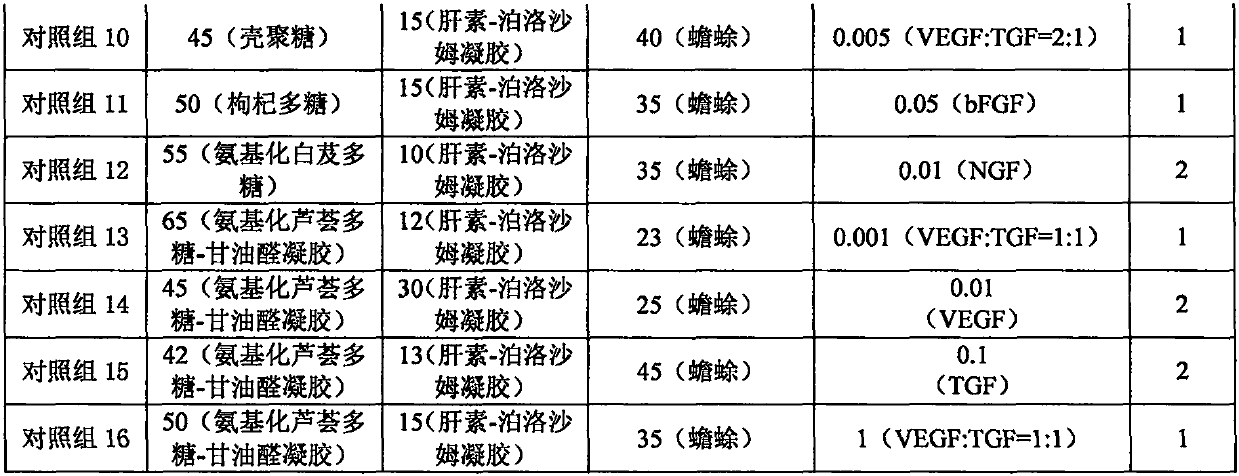
[0022] Example 1 Preparation of filling material for bone defect with low immunogenicity
[0023] Preparation method 1: According to the composition of each experimental group of filling materials for low-immunogenic bone defects in Table 1, weigh each component, crush the bone decellularized matrix according to the aseptic method, and mix with heparin-loaded high-affinity cells The heparin-poloxamer gel of the growth factor was mixed evenly, and the aminated aloe polysaccharide-glyceraldehyde gel was added, and mixed evenly to form a uniform semi-solid gel.
[0024] Preparation method 2: According to the composition of each experimental group of filling materials for low-immunogenic bone defects in Table 1, weigh each component, crush the bone decellularized matrix according to the aseptic method, and coagulate with aminated aloe polysaccharide-glyceraldehyde Mix the gel evenly, add heparin-poloxamer gel loaded with heparin high-affinity cell growth factor, and mix evenly to ...
Embodiment 2
[0033] Example 2 In vivo evaluation of the application effect of bone defect filling materials in each group
[0034] (1) Animal model of rabbit radius defect
[0035] The degree of bone defect in the bone defect model is based on the concept of critical bone defect proposed by Schmitz et al., that is, when the bone defect reaches 1.5 times the diameter of the long bone, the body cannot heal itself, resulting in bone defect, while the bone defect smaller than this multiple can heal itself .
[0036] Rabbit radial defect model: New Zealand white rabbits weighing 2.5-3kg were taken. After anesthesia, the blood vessels and tendons at the radius were separated, the radius was exposed, the radius was sawn off until it was 1.5 times the section diameter, and the radial bone defect was confirmed by Micro-CT images. Whether the defect position is correct and whether the gap is neat.
[0037] (2) In vivo application effect of each group of filling materials
[0038] Using the rabbit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com