Freeze-dried powder of antitumor drug and preparation method thereof
A technology of anti-tumor drugs and freeze-dried powders, which is applied in the direction of anti-tumor drugs, drug combinations, freeze-dried delivery, etc., and can solve the problems of low dependence of tumors on angiogenesis, adverse reactions, and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
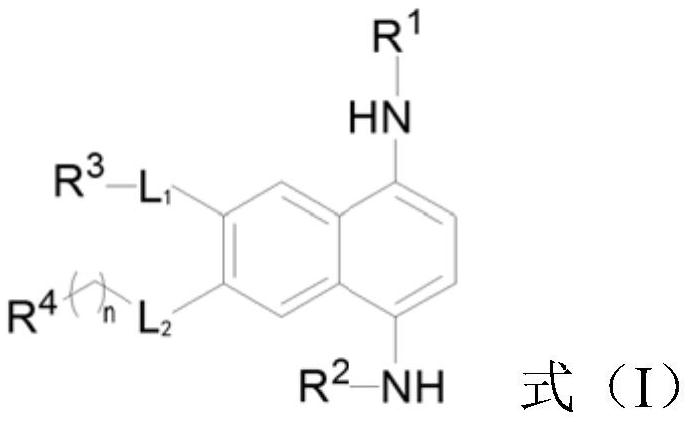
[0044] The preparation method of the 1,4-diaminonaphthalene derivative shown in formula (I) can comprise the following steps:
[0045] (a) making the compound shown in formula (1) carry out dealkylation reaction, obtain the compound shown in formula (2);
[0046] (b) making the compound shown in formula (2) carry out nucleophilic substitution reaction, obtain the compound shown in formula (3);
[0047] (c) In the presence of tert-butanol hydroperoxide and potassium hydroxide, a hydroxyl group is introduced into the compound shown in formula (3) to obtain a compound shown in formula (4);
[0048] (d) Substituting the compound shown in formula (4) to obtain the compound shown in formula (5);
[0049] (e) making the compound shown in formula (5) undergo an affinity substitution reaction with an amino substituent to obtain a compound shown in formula (6);
[0050] (f) making the compound shown in formula (6) undergo a hydrogenation reduction reaction to obtain the target compoun...
preparation example 1
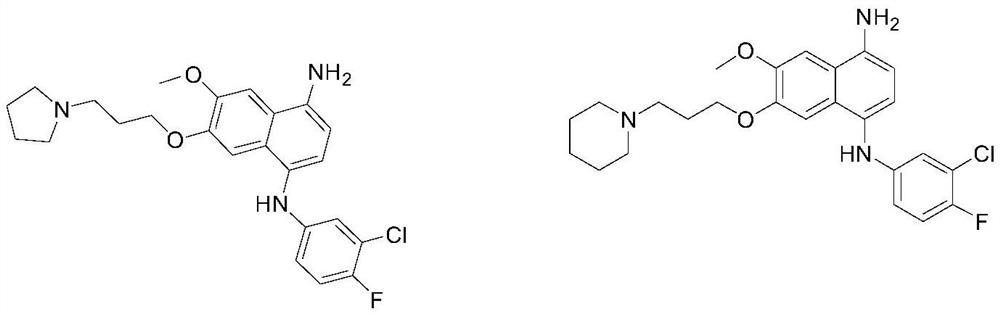
[0073] (1) Preparation of 3-methoxy-5-nitro-2-naphthol
[0074]
[0075] Dissolve 6,7-dimethoxy-1-nitronaphthalene (4.66g, 20mmol) and L-methionine (2.98g, 20mmol) in 38.4g of methanesulfonic acid (400mmol), and reflux the oil bath at 90°C Stir the reaction under heating conditions for 8 hours, after TLC detects that the reaction is complete, neutralize the reaction with a saturated sodium bicarbonate solution until no bubbles emerge, the pH test paper detects that it is neutral or weakly alkaline, and then extract the aqueous phase 3 times with ethyl acetate, The ethyl acetate phases were combined, washed three times with saturated sodium chloride, dried over anhydrous magnesium sulfate, filtered, evaporated to dryness under reduced pressure, and separated by column chromatography to obtain a yellow powdery solid (1.18 g, yield: 27%).
[0076] (2) Preparation of 4-(3-((3-methoxy-5-nitronaphthalene-2-) oxygen) propyl) morpholine
[0077]
[0078] Dissolve 3-methoxy-5...
Embodiment 1
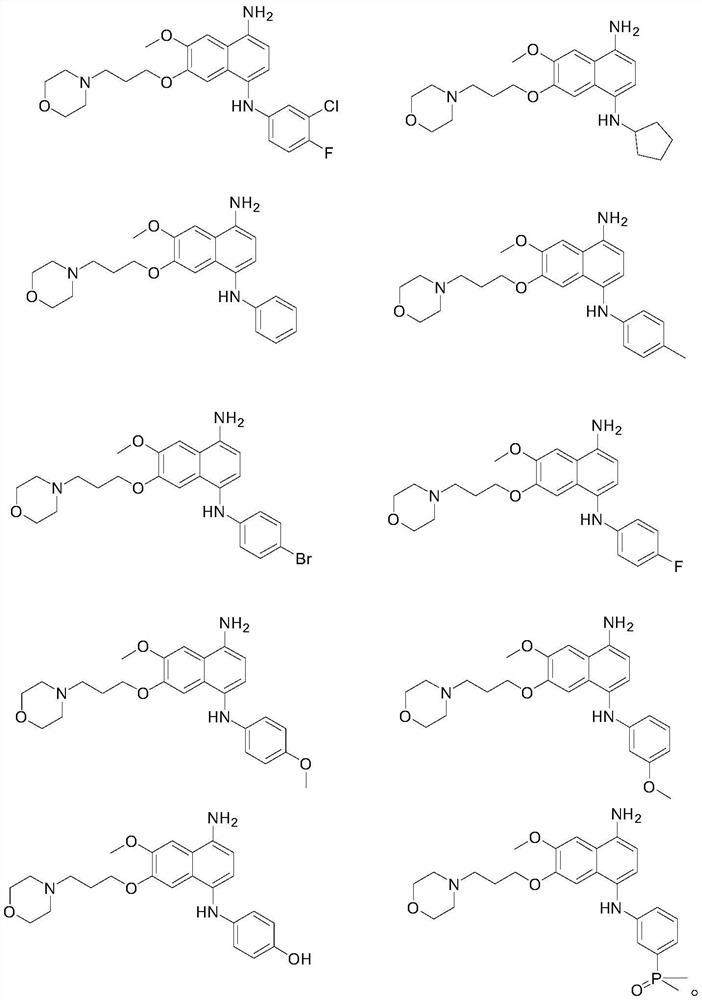
[0109] Under a sterile environment, accurately weigh 0.5g of compound 1 and 0.5g of mannitol, place them in a clean preparation container, add 4ml of water for injection, stir until completely dissolved, then add water for injection to 5ml, and stir evenly to obtain frozen Dry stock solution.
[0110] Fill the lyophilized stock solution into a 10ml control glass bottle, stopper halfway, and transfer it to a freeze dryer for freezing treatment. The specific freeze-drying process is as follows: the lyophilized stock solution is quickly frozen to -25°C for 2 hours, and then vacuumed to 15Pa. Raise the temperature to -5°C at a rate of 2°C per hour, sublimate and dry for 12 hours, then raise the temperature to 20°C at a rate of 8°C per hour, analyze and dry for 8 hours, fully stopper, and cap to obtain the antineoplastic drug jelly Dry powder A1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com