Application of Rspo1 to induce differentiation of bone marrow mesenchymal stem cells into cardiomyocyte-like cells
A technology of bone marrow mesenchymal and cardiomyocyte-like cells, applied in the field of regenerative medicine, can solve problems such as no reports on the effect, and achieve a significant effect of promoting differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Isolation and culture of rat BMSCs.
[0050] Under sterile conditions, expose the bone marrow cavity of the femur and tibia of the hindlimb of Sprague-Dawley rats, wash the bone marrow cavity with medium (L-DMEM + 10% fetal bovine serum + 1% double antibody, the same below) until the bone marrow cavity changes from red to White.
[0051] Pipette the bone marrow suspension into the 25cm 2 Culture bottle, placed in humidity ≥ 95%, 37°C, 5% CO 2 cultured in an incubator.
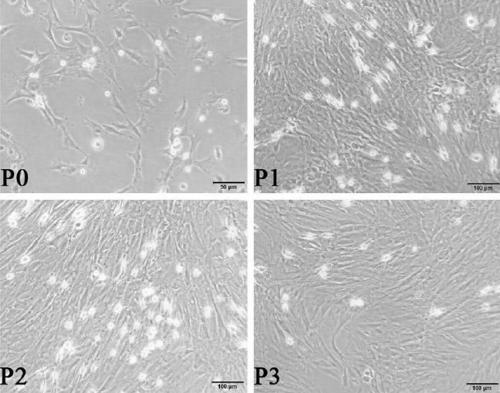
[0052] Three days later, when the confluence of BMSCs reached about 90%, the first passage was carried out. Discard the culture medium, wash it twice with PBS, add 1.5ml of 0.25% trypsin (containing EDTA) solution, digest at 37°C for 3 minutes, the cells become round and float under the microscope, add the culture medium to stop the digestion.
[0053] Transfer the cells into a centrifuge tube with a pipette and centrifuge at 1000rpm for 5min. Discard the supernatant, add the culture medi...
Embodiment 2
[0057] Example 2: Identification of rat BMSCs.
[0058] Take the P3 cells cultured in Example 1, add 0.25% trypsin without EDTA, digest at 37°C, and wash with PBS. The obtained cells were used to detect the specific surface molecules and cell cycle of BMSCs by flow cytometry.
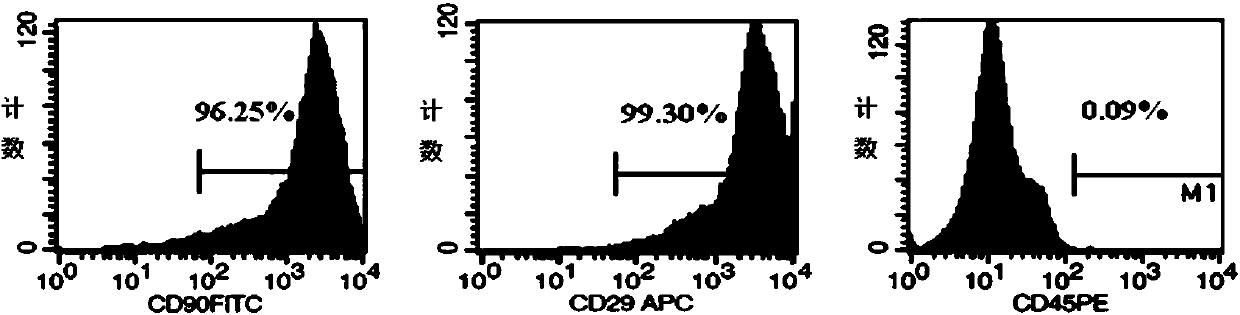
[0059] Prepare the cell suspension with 1ml PBS and count the cells so that the cell density is 1×10 5 cells / ml, centrifuge at 1000rpm for 5min, discard the supernatant. Cell suspension was prepared in 200 μl PBS, CD29-APC, CD90-FITC, CD45-PE antibodies (Abcam Company) were added, and incubated at room temperature for 30 min in the dark. Wash with PBS, resuspend the cells in 200 μl buffer, and detect the specific surface molecules CD29, CD90 of BMSCs and CD45 of hematopoietic cells by flow cytometry, and the results are as follows figure 2 shown.
[0060] figure 2 It shows that the expression rates of BMSCs-specific surface molecules CD29 and CD90 are (99.54±0.13)% and (95.74±1.39)% respectively,...
Embodiment 3
[0069] Example 3: Using adenovirus as a carrier Rspo1 Rat BMSCs were transfected.
[0070] The P3 cells of Example 1 were taken, washed with PBS, added with 0.25% trypsin containing EDTA, and digested at 37°C for 3 minutes. Cells in 8×10 4 The density of each well is planted in a 6-well plate, the humidity is ≥95%, 37 ° C, 5% CO 2 Cultivate in the incubator for 24h.
[0071] Divide BMSCs into 3 groups: control group, only add 2ml of medium without double antibody; negative control group, add 1ml of negative control virus solution + 1ml of medium without double antibody; Rspo1 overexpression group, add concentration of 2×10 8 PFU / ml with gene Rspo1 1ml of virus solution + 1ml of double antibody-free medium.
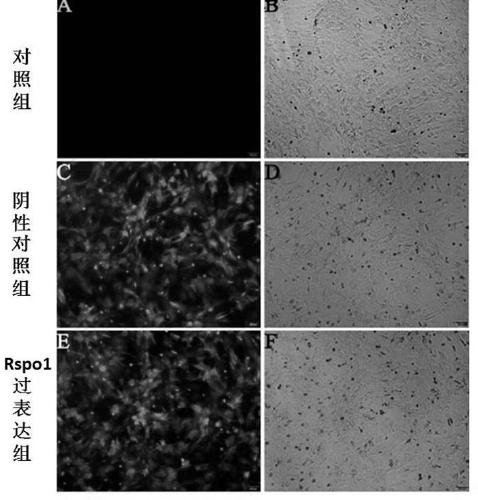
[0072] The three groups of BMSCs were incubated for 24 hours, then the medium was changed, and the incubation was continued for 2 days, observed and photographed with a fluorescence microscope.
[0073] according to Image 6 Fluorescence microscope observation resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com