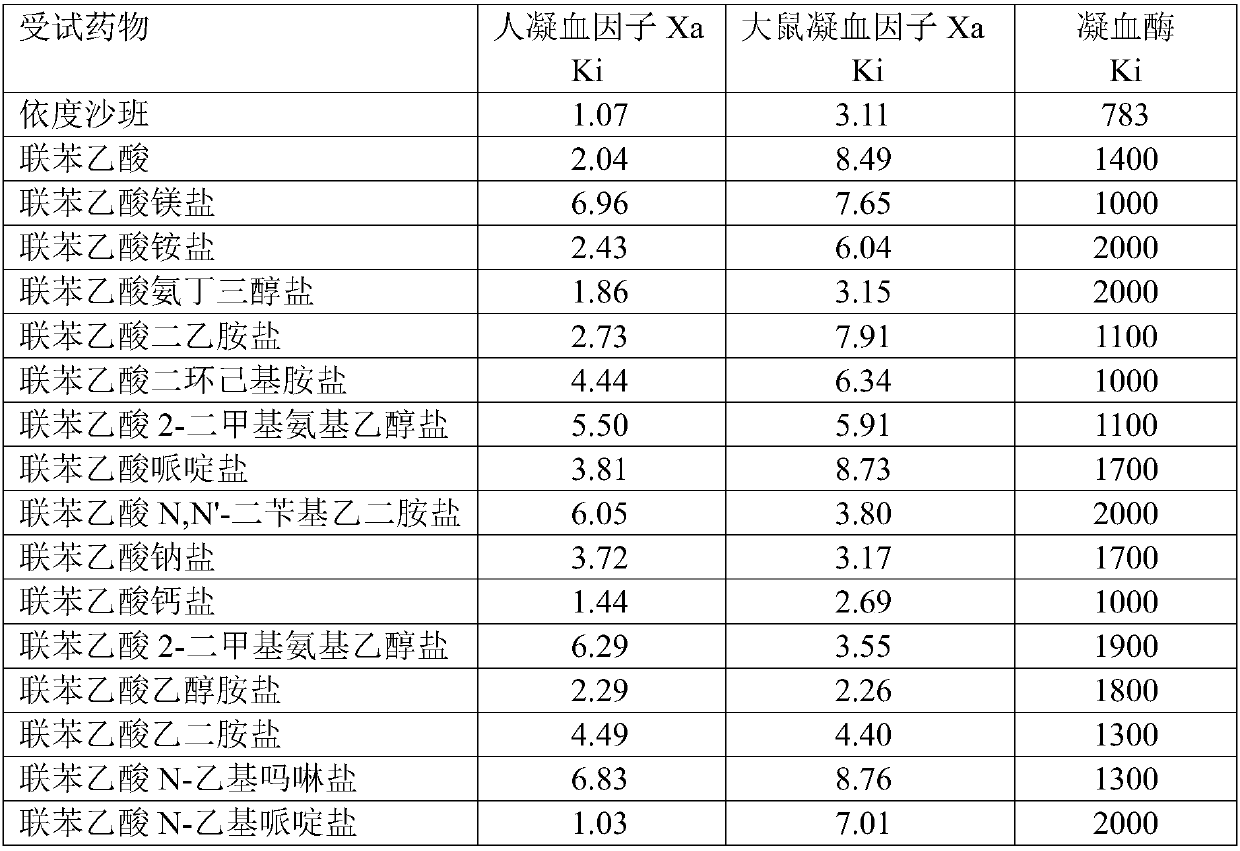
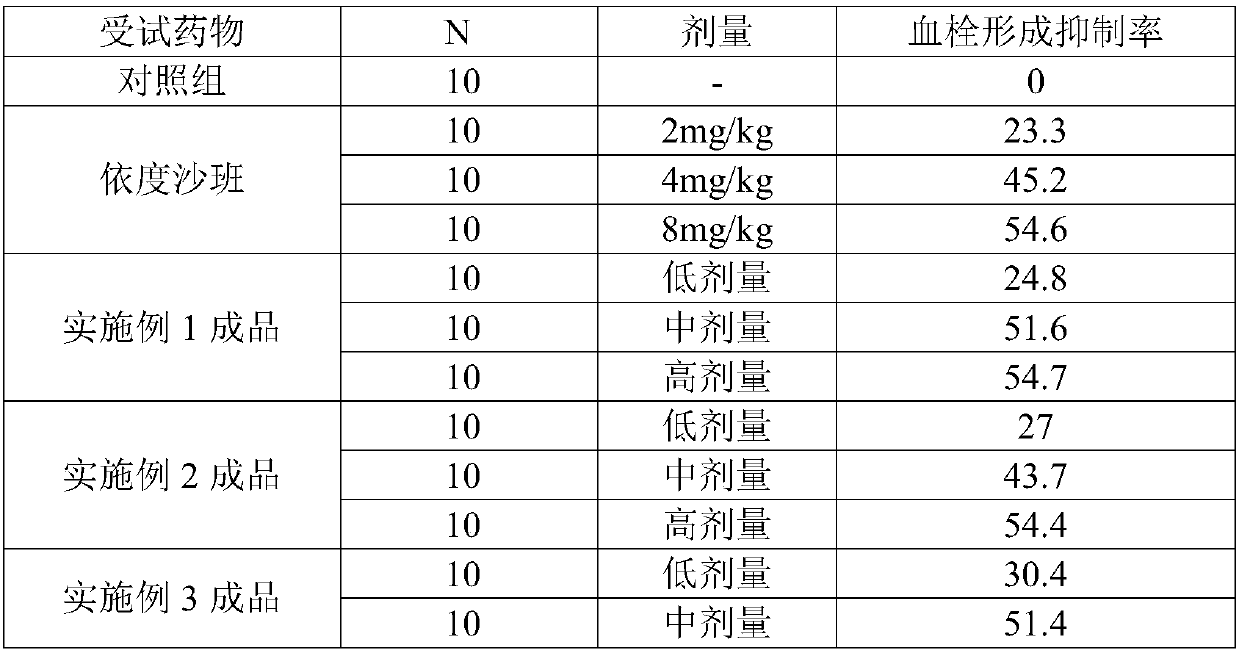
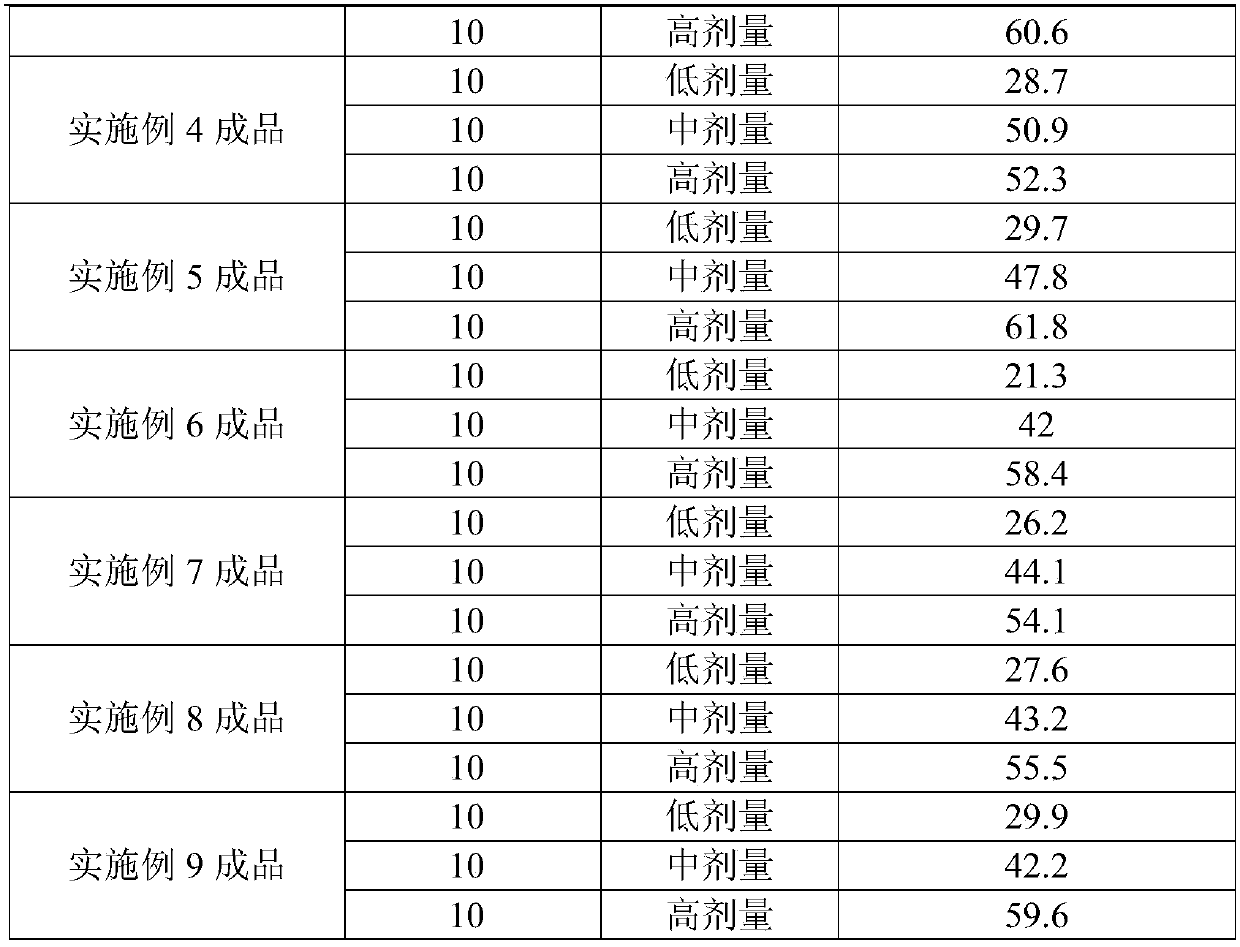
Application of biphenylacetic acid or salt thereof to preparation of Xa inhibitor medicament
A kind of technology of felbinac ammonium salt and felbinac manganese salt, applied in the field of new use of felbinac, can solve the problems such as reports of felbinac that have not been found yet, and achieve the effect of preventing cerebral infarction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: felbinac dry suspension
[0041] Preparation formula: 30 grams of felbinac, 670 grams of sucrose powder, 20 grams of sodium alginate, 3 grams of stevia, 15 grams of hydroxypropyl cellulose, 3 grams of sodium lauryl sulfate, 0.1 grams of sunset yellow, carboxymethyl Sodium starch starch 15 grams.
[0042] Preparation:
[0043] 1. Prepare each component according to the formulation of the preparation and crush it;
[0044] 2. Take 1 gram of steviol, 0.05 gram of sunset yellow, 3 grams of sodium lauryl sulfate, 30 grams of felbinac, and 500 grams of sucrose powder, mix them in equal increments, and granulate with 25ml of 20% ethanol aqueous solution , to obtain loose granules and powders that form into agglomerates when held by hand, loose by hand, and have rough edges, which is part A, with a total of 548 grams;
[0045] 3. Take the remaining 2 grams of stevia, 0.05 grams of sunset yellow, 170 grams of sucrose powder, 15 grams of hydroxypropyl cellulose, 15...
Embodiment 2
[0048] Embodiment 2: Felbinac tromethamine salt injection
[0049] Raw materials: 12.0 g of felbinac tromethamine salt, 1000 ml of water for injection, anhydrous sodium carbonate solution, and adjust the pH value to 8.2.
[0050] Preparation:
[0051] Take by weighing 12.0g of felbinac tromethamine salt, add 1000ml of cooled water for injection, dissolve in an appropriate amount of 0.1mol / L anhydrous sodium carbonate solution, add 0.1% activated carbon for needles (ie 0.8g) according to the total amount prepared , keep stirring at 60°C for 15 minutes, filter and decarburize, cool to room temperature, add cooled water for injection, adjust the pH value to 8.2, stir evenly, filter through a 0.22 μm microporous membrane, and seal in a 5ml vial. Each bottle was filled with 4ml, sealed, and sterilized by autoclaving at 121°C for 15 minutes to obtain a total of 232 finished products.
Embodiment 3
[0052] Embodiment 3 felbinac magnesium salt tablet
[0053] Raw materials: magnesium felbinac 50g, sodium carboxymethyl starch 9g, microcrystalline cellulose 22.5g, lactose 115.5g, povidone K 30 1.5g, micropowder silica gel 1.5g.
[0054] Preparation:
[0055] Weigh 50g of magnesium felbinac, 9.0g of sodium carboxymethyl starch, 22.5g of microcrystalline cellulose, 115.5g of lactose, and povidone K that were crushed through a 200-mesh sieve. 30 1.5g, micropowder silica gel 1.5g, fully mix magnesium felbinac, sodium carboxymethyl starch, microcrystalline cellulose, lactose, and mix povidone K 30 Dissolve it in 30ml of pure water as a binder to make a soft material, granulate it with a 18-mesh sieve, and dry it at 60°C. The dry granules pass through a 16-mesh sieve for granulation, add micropowder silica gel and mix well, and adjust the tablet weight to 200 mg / Tablets can be obtained by pressing tablets, and a total of 957 finished products are made.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com