Method for preparing SiF4 and anhydrous hydrogen fluoride from fluosilicic acid
An anhydrous hydrogen fluoride and fluosilicic acid technology, applied in fluorine/hydrogen fluoride, hydrogen fluoride, chemical instruments and methods, etc., can solve the problems of difficulty in obtaining purity, low concentration of fluosilicic acid, difficult liquid transportation, etc., and achieve added value of products The effect of high, high technical content and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A kind of preparation SiF with fluosilicic acid as raw material 4 and the method for anhydrous hydrogen fluoride, comprising the following steps:
[0028] (1) Preparation of ammonium fluorosilicate: add liquid ammonia to fluorosilicic acid, neutralize until the pH is 3.2, filter, concentrate and crystallize the filtrate, and dry to obtain ammonium fluorosilicate;
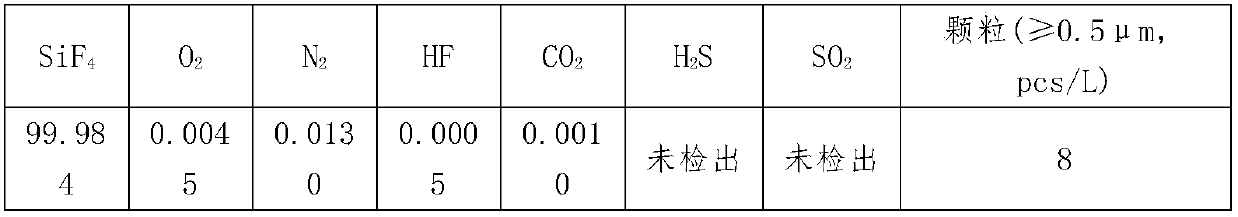
[0029] (2) The first pyrolysis: After grinding the ammonium fluorosilicate, add concentrated sulfuric acid, stir evenly, the liquid phase enters the airtight container for pyrolysis reaction, the temperature is 80°C, the time is 30min, the escaped gas phase is treated with concentrated sulfuric acid Carry out washing, the washing temperature is 30°C, the washed gas is cooled to -10°C, and the alumina and silica gel packing columns are used for secondary adsorption, and the adsorption time of each level is 2min to obtain SiF 4 The gas is frozen at -90°C to produce the SiF gas for the electronic industry that ...
Embodiment 2
[0038] A kind of preparation SiF with fluosilicic acid as raw material 4 and the method for anhydrous hydrogen fluoride, comprising the following steps:
[0039] (1) Preparation of ammonium fluorosilicate: add liquid ammonia to fluorosilicic acid, neutralize until the pH is 3.5, filter, concentrate and crystallize the filtrate, and dry to obtain ammonium fluorosilicate;
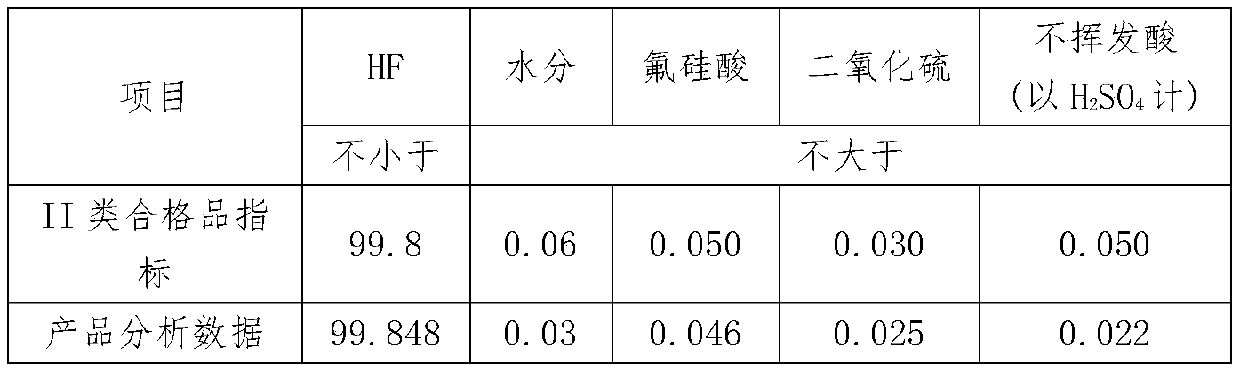
[0040] (2) The first pyrolysis: After grinding the ammonium fluorosilicate, add concentrated sulfuric acid, stir evenly, the liquid phase enters the airtight container for pyrolysis reaction, the temperature is 100°C, the time is 60min, and the escaped gas phase is treated with concentrated sulfuric acid Carry out washing, the washing temperature is 50°C, the gas after washing is cooled to 0°C, and the alumina and silica gel packing column is used for secondary adsorption, and the adsorption time of each level is 5min to obtain SiF 4 Gas, compressed and liquefied to produce high-purity SIF 4 gas;
[0041] ...
Embodiment 3
[0049] A kind of preparation SiF with fluosilicic acid as raw material 4 and the method for anhydrous hydrogen fluoride, comprising the following steps:
[0050] (1) Preparation of ammonium fluorosilicate: add liquid ammonia to fluorosilicic acid, neutralize to pH 3.4, filter, concentrate and crystallize the filtrate, and dry to obtain ammonium fluorosilicate;
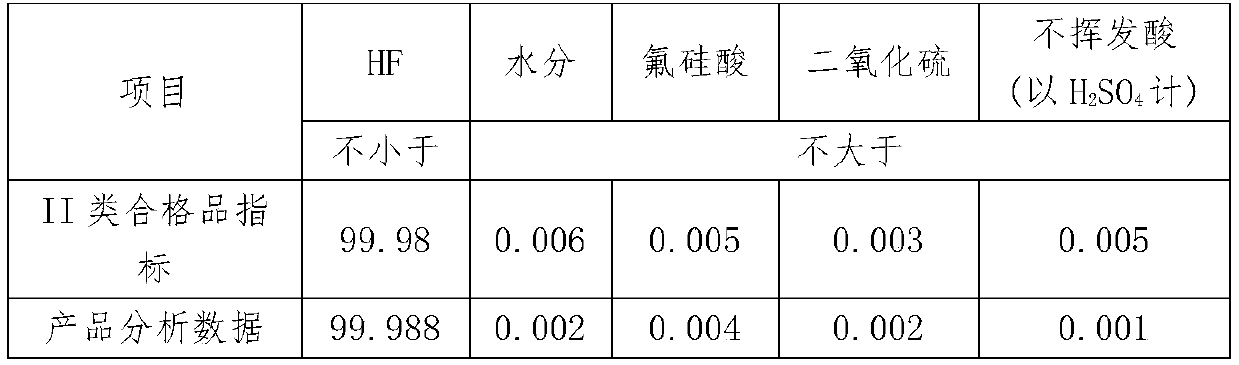
[0051] (2) The first pyrolysis: After grinding the ammonium fluorosilicate, add concentrated sulfuric acid, stir evenly, the liquid phase enters the airtight container for pyrolysis reaction, the temperature is 90°C, the time is 45min, the escaped gas phase is treated with concentrated sulfuric acid Carry out washing, the washing temperature is 40°C, the washed gas is cooled to -5°C, and the alumina and silica gel packing columns are used for secondary adsorption, and the adsorption time of each level is 3min to obtain SiF 4 The gas is frozen at -70°C to produce the SiF gas used in the electronics industry that meets th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com