Modified agarase and application thereof
A technology of agarase and new agar oligosaccharides, which is applied in the field of enzyme engineering, can solve the problems that new agar oligosaccharides with a low degree of polymerization cannot be obtained in large quantities, and achieve the effects of convenient control, low requirements, and high utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Transformation and recombinant expression of agarase gene
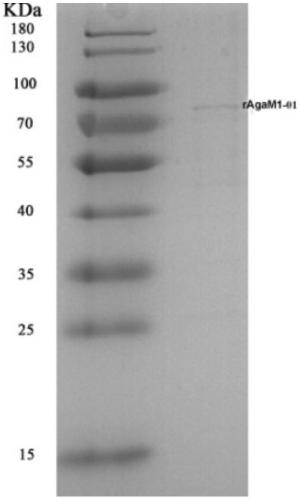
[0035]Bioinformatics analysis and function prediction were carried out on the existing agarase gene. The structure of the original agarase gene was predicted by NCBI-BLAST, and the results showed that there was a homologous sequence of the Porsecretion system C-terminal sorting domain in the amino acid sequence corresponding to the 5' end of the gene. By PCR technology combined with primer design, the nucleotide sequence of the region corresponding to the domain at the 3' end of the existing agarase AgaM1 gene is truncated to obtain the artificially truncated agarase gene AgaM1-01 (SEQ ID NO: 2 ). AgaM1-01 was connected into the expression vector pEASY-Blunt E1 to obtain the recombinant vector pEASY-AgaM1-01, and the recombinant vector was transformed into Escherichia coli BL21 (DE3) strain;
[0036] Insert the recombinant Escherichia coli strain into LB liquid medium, cultivate at 37°C until th...
Embodiment 2
[0037] Example 2: Optimization of production conditions for new agar oligosaccharides
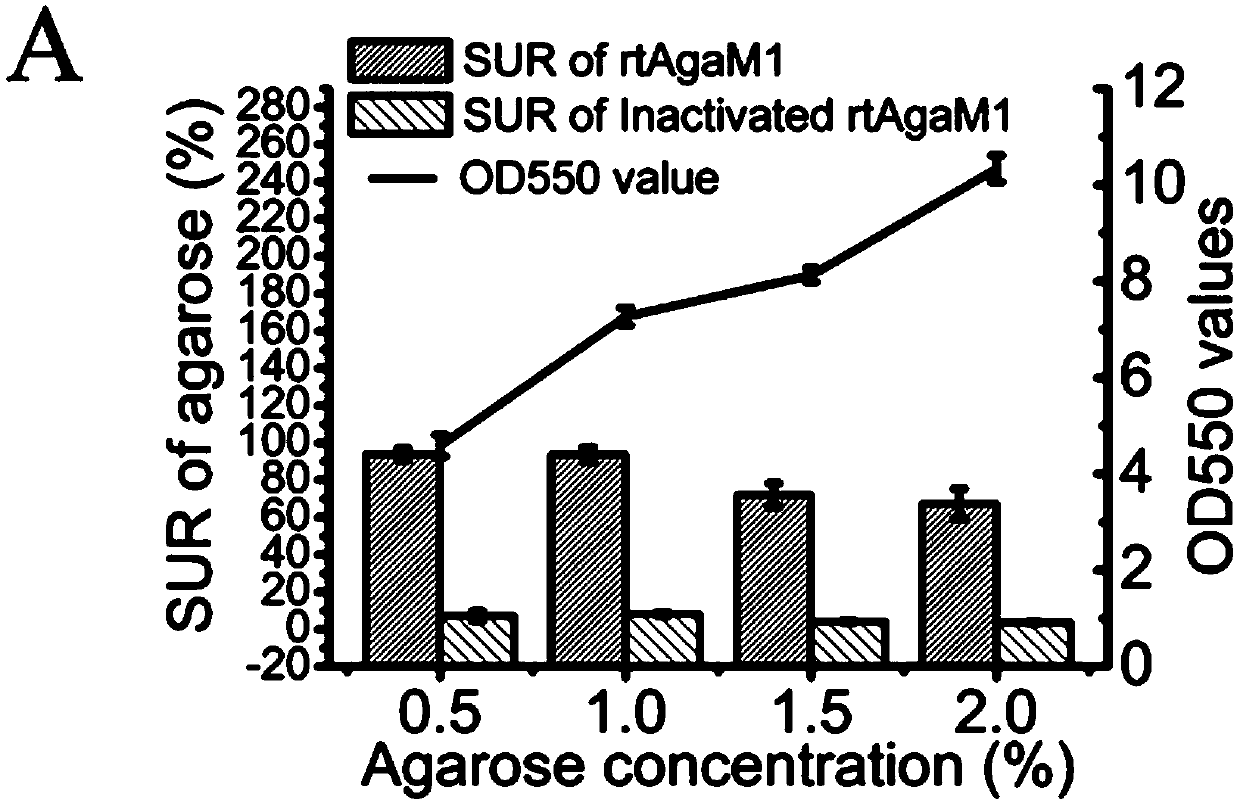
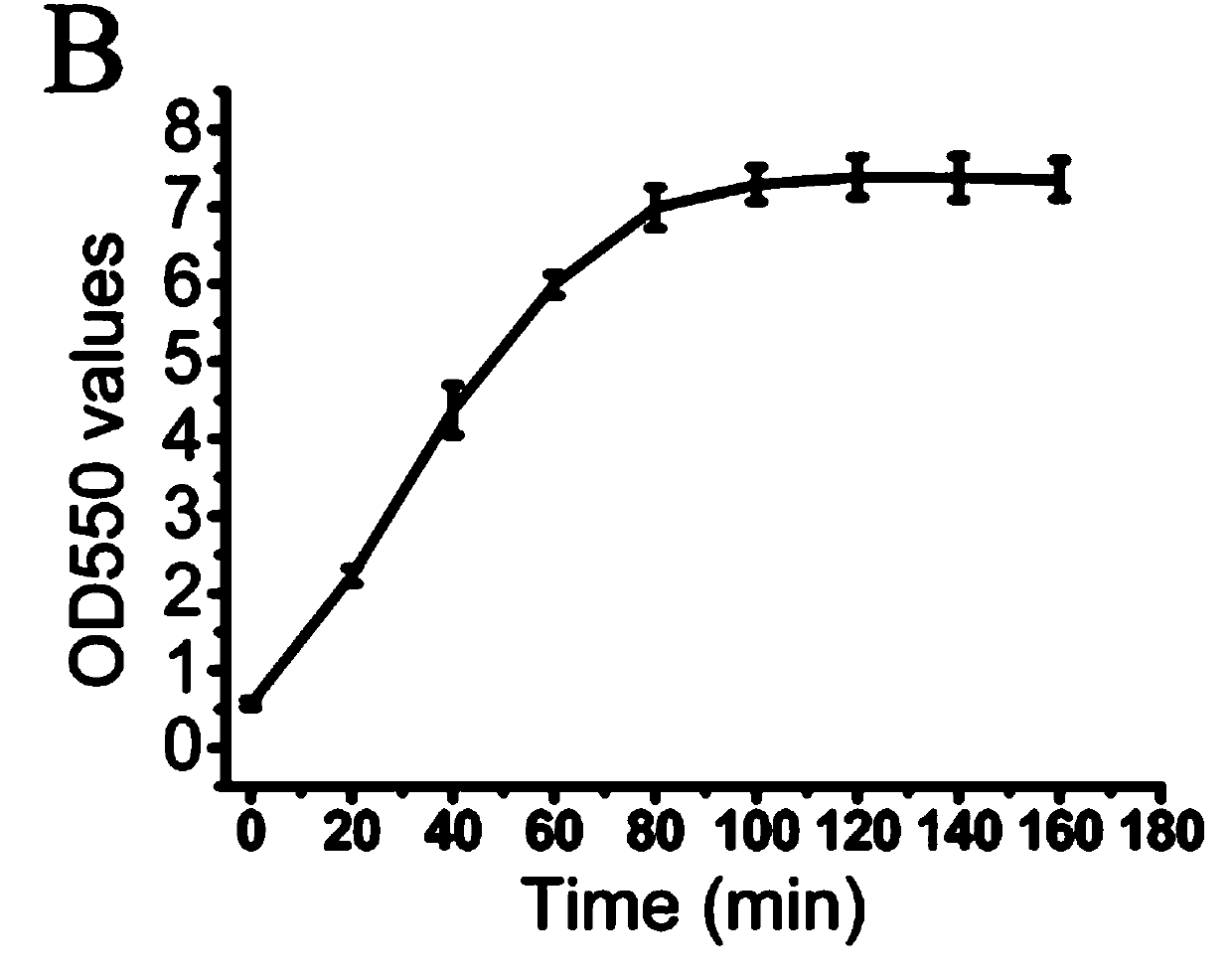
[0038] Agar concentration optimization. Theoretically, the higher the agar concentration, the higher the yield of oligosaccharides. However, as the reaction proceeds, the activity of the enzyme begins to decrease, eventually resulting in part of the agar not participating in the reaction, and this phenomenon is more common in high-concentration agar. The unreacted agar solidifies into a solid as the temperature decreases after the reaction, and these colloidal solids will block the pipelines and filter membranes of the production equipment, resulting in waste of substrates, loss of equipment, and additional labor costs. Therefore, in the production process, the substrate utilization rate is the primary optimization factor considered. Enzyme activity was measured by the dinitrosalicylic acid method, and the substrate utilization ratio (substrate utilization ratio=(initial reaction system q...
Embodiment 3
[0041] Embodiment 3: Composition analysis of new agar oligosaccharide product
[0042] Degradation products and polymerization degrees of the original agarase (AgaM1) and the truncated agarase (AgaM1-01) used in this paper were determined by thin layer chromatography (TLC). As a result, it was found that the original agarase AgaM1 could only produce new agar oligosaccharides (new agarose and new agarosexose) with a low degree of polymerization ( Figure 3A ), and the truncated agarase (AgaM1-01) can obtain new agar oligosaccharides with a degree of polymerization of 4-12 under the above-mentioned optimal conditions ( Figure 3B ). The above results show that: 1) through artificial modification, the original agarase obtained new enzymatic properties after being truncated artificially; 2) the above-mentioned optimum production conditions are effective, and the Under the above optimal conditions, new agar oligosaccharides with a degree of polymerization of 4-12 can be obtained,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com