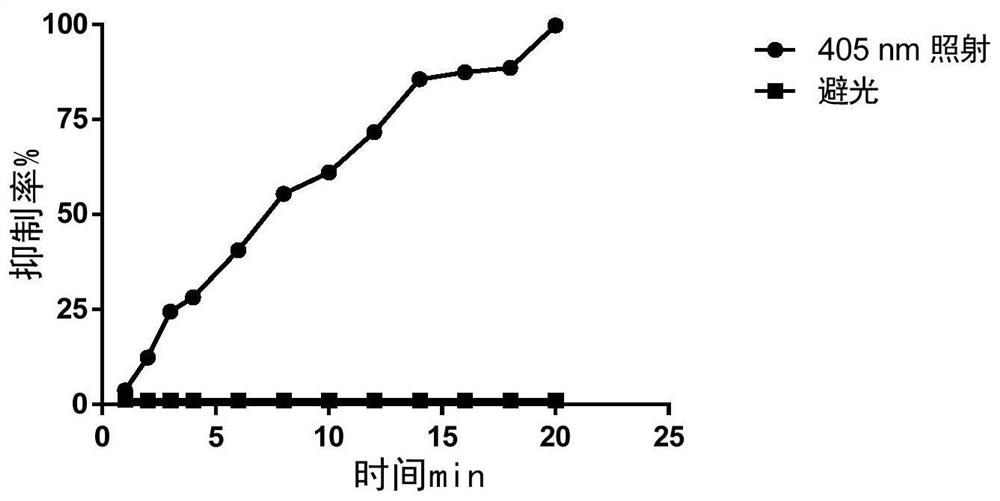
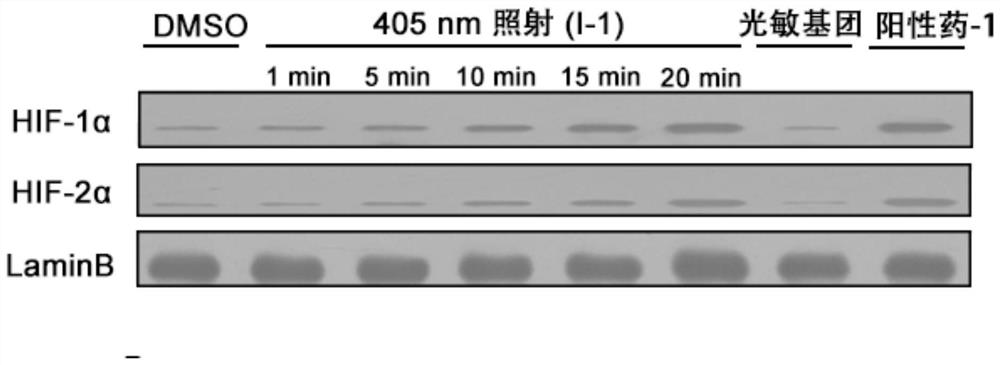
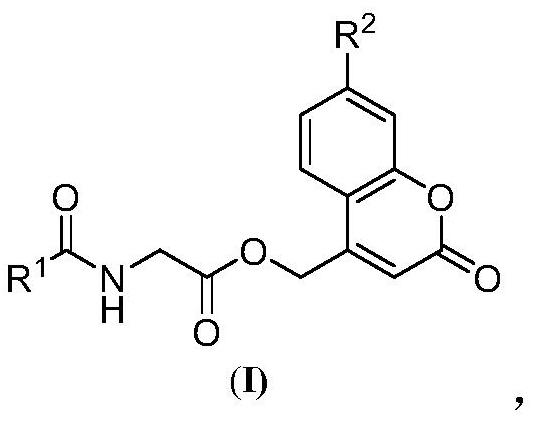
Prolyl hydroxylase small molecule photosensitive prodrug and its preparation method and application
A prolyl hydroxylase and small molecule technology, applied in the field of chemical biology, can solve the problems of complexity and poor bioavailability, and achieve the effects of improved selectivity, reasonable design and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of fluorescent probe Ⅰ-1
[0038] N-(5-(1-(2-(4-chlorophenoxy)ethyl)-1H-1,2,3-triazol-4-yl)-3-hydroxypicolinate)glycine (0.834 g, 2.0mmol), 4-(bromomethyl)-7-(diethylamino)-2H-pyran-2-one (0.494g, 2.0mmol), and potassium fluoride (0.174g, 3.0mmol) Dissolved in DMF (6mL), under N 2 The mixture was stirred at 45 °C for 2.0 h under protection and washed with CH 2 Cl 2 (3 x 5 mL) extraction. The combined organic phases were washed twice with saturated NaCl water (5 mL), anhydrous NaCl2 SO 4 Dry, filter, concentrate in vacuo, and purify the crude product by column chromatography (CH 2 Cl 2 : EtOAc=3:8), the target product probe Ⅰ-1 0.641g was obtained, the yield was 49.7%, R f : 0.31 (methanol:ethyl acetate=2:5), m.p.250.7-253.1°C, the compound 1 H-NMR (300MHz, DMSO-d 6 )δ12.29(s,1H),9.62(s,1H),8.92(s,1H),8.70(s,1H),7.83(s,1H),7.43(d,J=7.9Hz,1H), 7.31(d, J=7.1Hz, 2H), 6.98(d, J=7.0Hz, 2H), 6.58(d, J=7.8Hz, 1H), 6.50(s, 1H), 6.06(s, 1H), 5.36(s,2H),4.84...
Embodiment 2
[0040] Preparation of fluorescent probe Ⅰ-2
[0041] The preparation method is the same as in Example 1, replacing (4-(bromomethyl) with 4-(hydroxymethyl)-7-(piperidin-1-yl)-2H-chromen-2-one (0.518g, 2.0mmol) )-7-(diethylamino)-2H-pyran-2-one, to obtain yellow solid 0.716g, yield 54.4%, R f : 0.32 (methanol:ethyl acetate=2:5), m.p.253.1-255.6°C, the compound 1 H NMR (500MHz, DMSO-d 6 )δ8.73(d, J=1.6Hz, 1H), 8.41–8.34(m, 1H), 8.12(s, 1H), 7.86(d, J=1.5Hz, 1H), 7.52(d, J=7.5 Hz,1H),7.29–7.23(m,2H),6.98–6.93(m,2H),6.76(dd,J=7.4,1.6Hz,1H),6.59(d,J=1.5Hz,1H),6.14 (t,J=1.0Hz,1H),5.28(d,J=1.1Hz,2H),4.54–4.48(m,2H),4.46–4.31(m,2H),4.17(d,J=9.7Hz, 2H), 3.42(t, J=6.9Hz, 4H), 1.72–1.56(m, 6H)., HRMS(ESI):found659.1949(C 33 h 31 ClN 6 o 7 ,[M+H]+,requires 659.1943).
Embodiment 3
[0043] Preparation of fluorescent probe Ⅰ-3
[0044] The preparation method is the same as in Example 1, replacing N-(5-(1-(2-(4-chloro Phenoxy)ethyl)-1H-1,2,3-triazol-4-yl)-3-hydroxypyridinecarboyl)glycine, with 2,2'-((4-(bromomethyl)- 2-Oxo-2H-chromen-7-yl)azadiyl)diacetic acid (0.742 g, 2.0 mmol) in place of 4-(bromomethyl)-7-(diethylamino)-2H-pyran -2-ketone, 0.790g of yellow solid was obtained, the yield was 66.7%, m.p.260.1-263.6°C, the compound 1 H NMR (500MHz, DMSO-d 6 )δ8.45(d, J=1.6Hz, 1H), 8.37(t, J=9.6Hz, 1H), 7.67(d, J=1.4Hz, 1H), 7.64–7.58(m, 2H), 7.50( d,J=7.5Hz,1H),7.39–7.36(m,1H),7.35(dt,J=7.5,1.7Hz,1H),6.88(dd,J=7.5,1.5Hz,1H),6.64(d , J=1.4Hz, 1H), 6.14(t, J=0.9Hz, 1H), 5.28(d, J=1.1Hz, 2H), 4.19–4.13(m, 6H)., HRMS(ESI): found 595.1004 (C 28 h 22 ClN 3 o 10 ,[M+H] + ,requires 596.0994).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com