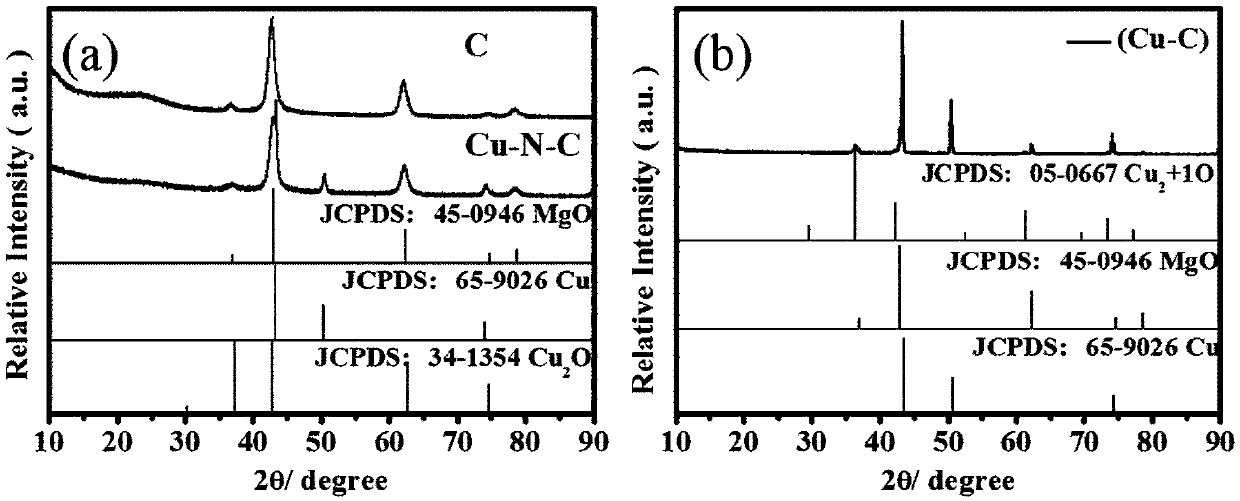
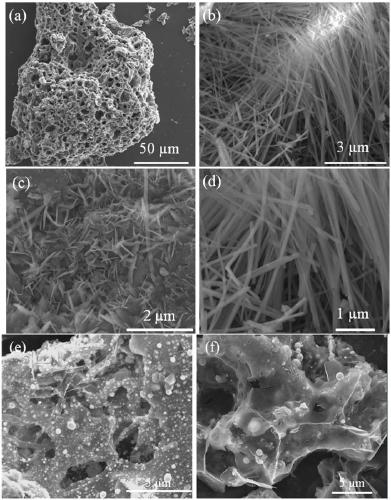
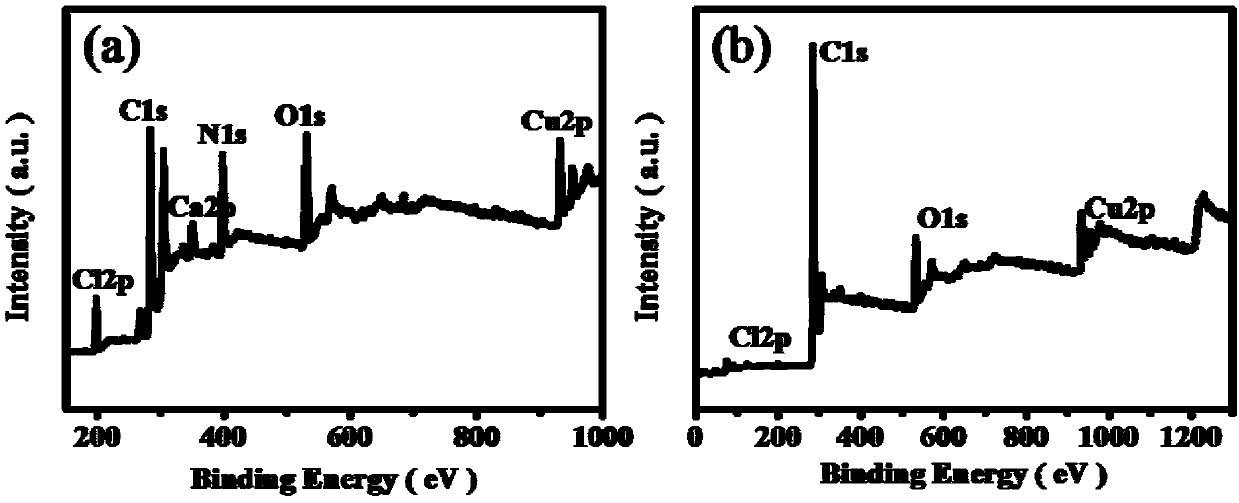
Copper-doped nitrogen-modified carbon material as well as application thereof and reaction method for preparing symmetric/asymmetric azobenzenes by oxidative coupling of aromatic amines
A technology of copper doping and carbon materials, which is applied in the field of carbon materials modified by copper doping and nitrogen, and the oxidative coupling of aromatic amines to azobenzene, which can solve the problems of poor selectivity, low product yield, unhealthy environment and human body, etc. Achieve strong substrate compatibility and good catalytic ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of copper-doped nitrogen-modified carbon materials:
[0031] Add 0.04mol of citric acid and 0.02mol of magnesium nitrate into a 500ml beaker, add 20ml of deionized water, fully dissolve, and vibrate for 10min in a 90Hz power ultrasonic cleaner to make the solution clear. Place the beaker open in a blast oven at 120°C and keep it under this condition for 24h. After the end, close the oven, cool to room temperature and take out the yellow foamy solid (precursor of the carrier). According to the mass ratio of precursor: melamine: copper salt, which is 4:4:3, the reagent is weighed and ground in an agate mortar until uniform. After transferring to a ceramic dry pot, put it in an atmosphere furnace N 2 Under protected conditions, keep warm at 800°C for 1h (heating rate 5°C / min), cool to room temperature after sintering, take it out and grind it evenly in an agate mortar to obtain a black solid, that is, Cu-N-C catalyst (copper-doped nitrogen-modified carbon mate...
Embodiment 2
[0034] The difference from Example 1 is that CuCl is selected as the copper salt, and the synthesized Cu-N-C (Cu, 8mol%) type material is used as the catalyst to catalyze the aniline reaction, and the separation yield is 44%.
Embodiment 3
[0036] The difference from Example 1 is that CuBr is selected as the copper salt, and the synthesized Cu-N-C (Cu, 8mol%) type material is used as the catalyst to catalyze the aniline reaction, and the separation yield is 13%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com