New green synthesis method for efficient synthesis of isoquinoline derivatives by transition metal catalyzed C-H activation/cyclization reaction
A technology of transition metal catalysis and cyclization reaction, applied in the direction of organic chemistry, can solve the problems of lengthy steps, environmental pollution, high cost, etc., and achieve the effect of simple steps and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Implementation Example 1: Synthesis of Compound 1
[0022]
[0023] (1) In a clean reactor, add benzylamine (21.4 mg, 0.2 mmol), 2,6-dimethoxyphenylthioylide (102.5 mg, 0.4 mmol), dichloro(pentamethylcyclopentadiene) Alkenyl) rhodium (III) dimer (6.18 mg, 0.01 mmol), silver acetate (6.67 mg, 0.04 mmol) and water (2 mL), put in an oil bath at 100°C and stir for 24 h.
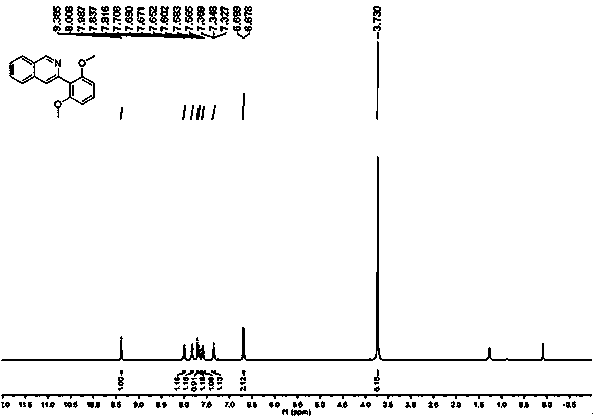
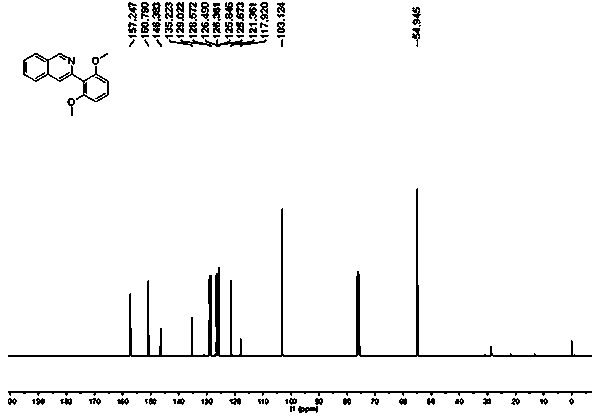
[0024] (2) After the reaction, dichloromethane was added for extraction, the dichloromethane layer was collected, the solvent was removed under reduced pressure, and the residue was separated and purified by silica gel column chromatography to obtain a white solid with a yield of 75%. 1 H NMR (400 MHz, CDCL 3 ) δ 9.39 (s,1H), 8.00 (d, J = 8.1 Hz, 1H), 7.83 (d, J = 8.2 Hz, 1H), 7.71 (s, 1H), 7.67(t, J = 7.6 Hz, 1H), 7.58 (t, J = 7.4 Hz, 1H), 7.35 (t, J = 8.4 Hz, 1H), 6.69(d, J = 8.4 Hz, 2H), 3.73 (s, 6H). 13 C NMR (101 MHz, CDCl 3 ) δ 157.25(s,2 C ), 150.79, 146.38, 135.22, 129.02, 128.5...
Embodiment 2
[0025] Implementation Example 2: Synthesis of Compound 2
[0026]
[0027] (1) Add p-bromobenzylamine (37.2 mg, 0.2 mmol), 2,6-dimethoxyphenylthioylide (102.5 mg, 0.4 mmol), dichloro(pentamethylcyclo Pentadienyl) rhodium (III) dimer (6.18 mg, 0.01 mmol), silver acetate (6.67 mg, 0.04 mmol) and water (2 mL), put in an oil bath at 100°C and stir for 24 h.
[0028] (2) After the reaction, dichloromethane was added for extraction, the dichloromethane layer was collected, the solvent was removed under reduced pressure, and the residue was separated and purified by silica gel column chromatography to obtain a white solid with a yield of 71.1%. 1 H NMR (400 MHz, CDCl 3 ) δ 9.34 (s,1H), 7.99 (d, J = 1.6 Hz, 1H), 7.86 (d, J = 8.8 Hz, 1H), 7.65 (dd, J = 8.8,1.6 Hz, 1H), 7.61 (s, 1H), 7.35 (t, J = 8.4 Hz, 1H), 6.68 (d, J = 8.4 Hz,2H), 3.73 (s, 6H). 13 C NMR (101 MHz, CDCl 3 ) δ 158.22 (s,2 C), 151.72 (s), 148.65 (s), 137.30 (s), 130.50 (s), 129.89 (s), 129.19 (s), 128.9...
Embodiment 3
[0029] Implementation Example 3: Synthesis of Compound 3
[0030]
[0031] (1) Add p-methylbenzylamine (24.2 mg, 0.2 mmol), 2,6-dimethoxyphenylthioylide (102.5 mg, 0.4 mmol), dichloro(pentamethyl Cyclopentadienyl) rhodium (III) dimer (6.18 mg, 0.01 mmol), silver acetate (6.67 mg, 0.04 mmol) and water (2 mL), were placed in an oil bath at 100°C and stirred for 24 h.
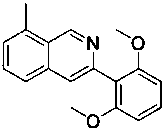
[0032] (2) After the reaction, dichloromethane was added for extraction, the dichloromethane layer was collected, the solvent was removed under reduced pressure, and the residue was separated and purified by silica gel column chromatography to obtain a light yellow solid with a yield of 56.4%. 1 H NMR (400 MHz, CDCl 3 ) δ 9.31 (s,1H), 7.89 (d, J = 8.4 Hz, 1H), 7.60 (d, J = 9.7 Hz, 2H), 7.42 (dd, J = 8.4,1.2 Hz, 1H), 7.34 (t, J = 8.4 Hz, 1H), 6.68 (d, J = 8.4 Hz, 2H), 3.73 (s,6H), 2.54 (s, 3H). 13 C NMR (101 MHz, CDCl 3 ) δ 157.26 (s,2 C ), 150.32 (s), 146.29 (s), 139.35 (s), 135.55 (s), 128.50 (s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com