Preparation method of pyrimidine derivative and application thereof
A technology of pyrimidine derivatives and reactions, applied in the field of new pyrimidine derivatives and preparation methods and their application in antitumor drugs, can solve the problem of low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
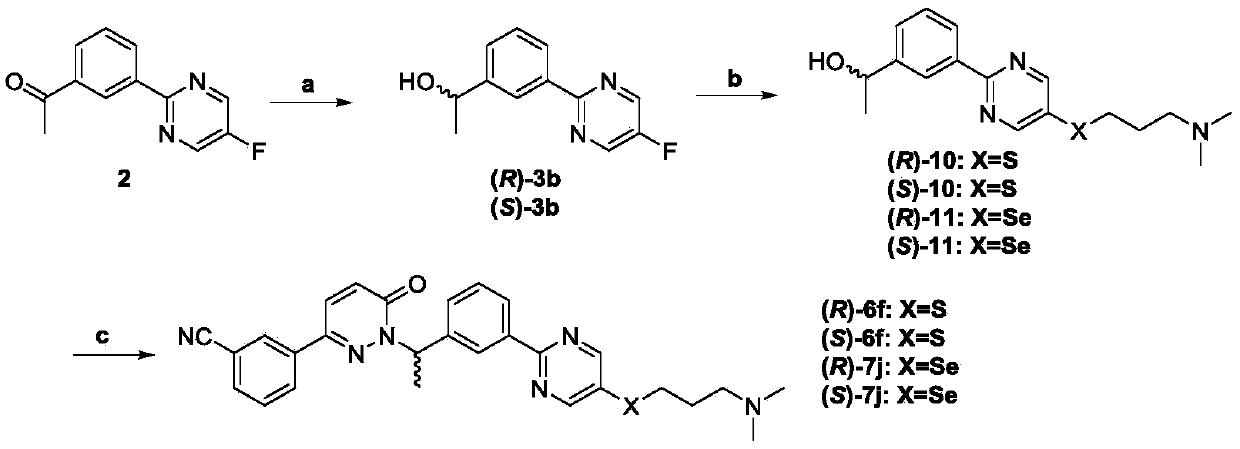
[0108] Embodiment 1: antitumor compound (±)-5b
[0109] Antitumor compound (±)-5b, its structural formula is as follows, and the synthesis steps are as follows:
[0110]
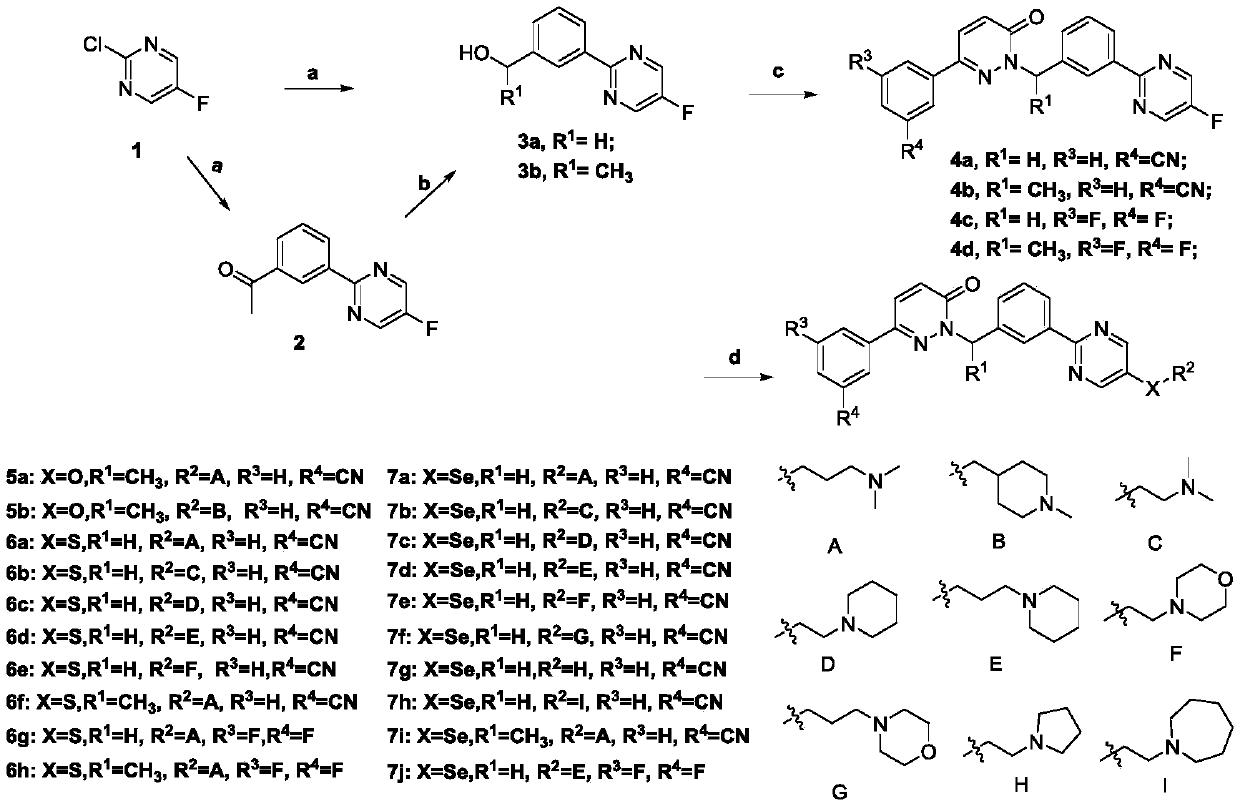
[0111] Preparation of Intermediate 2: Add 32 mL of toluene solution containing 5-fluoro-2-chloropyrimidine (33 mmol) to an aqueous solution of sodium carbonate (66 mmol) (32 mL of water), add bis(triphenylphosphine) palladium chloride ( 0.33mmol), and 65mL of an ethanol solution of acetylphenylboronic acid (32mmol) was added dropwise. The reaction was stirred at 80°C under nitrogen for 18 hours, cooled to room temperature, and filtered. Ethyl acetate and water were added to the filtrate, the organic phase was separated, and dried over anhydrous sodium sulfate. The crude product was separated by silica gel column chromatography (ethyl acetate:petroleum ether, 8:1) to obtain a light yellow solid, namely Intermediate 2. Yield 61%. 1 H NMR (400MHz, CDCl 3 )δ8.98 (t,J=1.6Hz,1H),8.70(s,2H),8.59(s,1H),8.09(s...
Embodiment 2
[0116] Embodiment 2: antitumor compound 6a
[0117] Antitumor compound 6a, its structural formula is as follows, and the synthesis steps are as follows:
[0118]
[0119] Preparation of Intermediate 3a: Sodium carbonate (66mmol) in water (32mL) was added to a toluene solution (32mL) containing 5-fluoro-2-chloropyrimidine (33mmol), bis(triphenylphosphine)palladium dichloride was added (0.33mmol), dropwise the ethanol (65mL) solution of hydroxymethylphenylboronic acid (32mmol), under nitrogen protection, stir at 80 ℃ for 18 hours, cool to room temperature, filter, add ethyl acetate and water in the filtrate, The organic phase was separated and dried over anhydrous sodium sulfate. The crude product was subjected to silica gel column chromatography (ethyl acetate:petroleum ether, 8:1), and a light yellow solid was obtained, namely intermediate 3a. The yield was 61%. 1 H NMR (400MHz, CDCl 3 )δ8.66 (d,J=1.8Hz,1H),8.37(d,J=4.9Hz,1H),8.31(s,1H),7.54-7.46(m,1H), 4.80(s,1H), 1.99(...
Embodiment 3
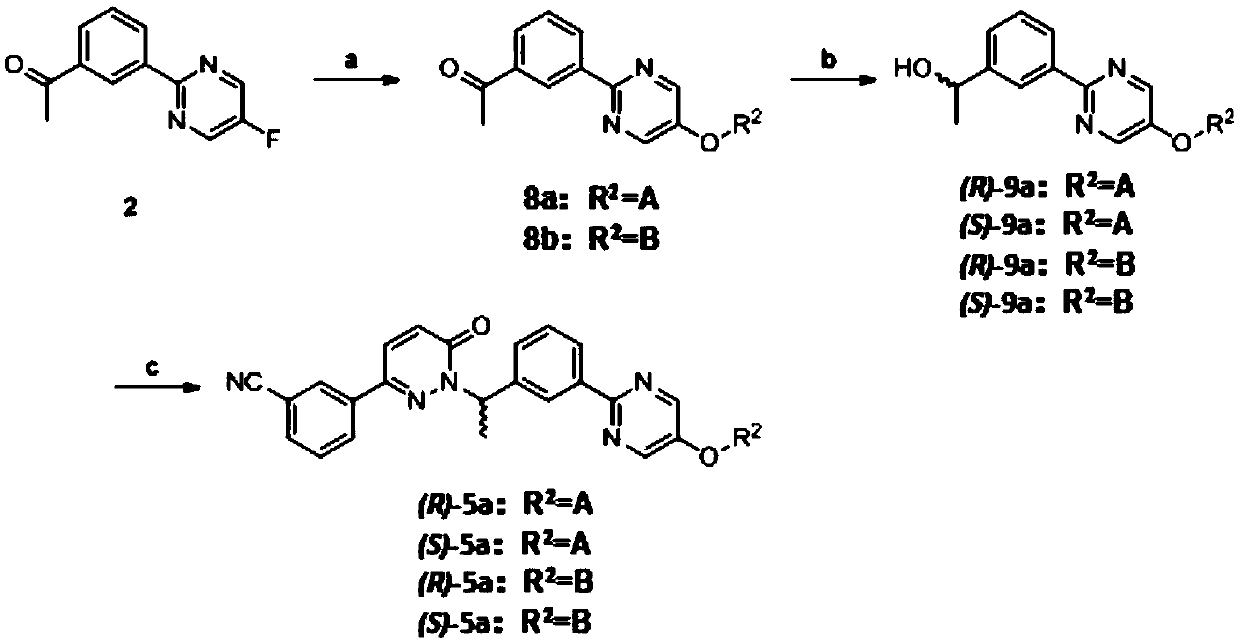
[0123] Embodiment 3: antitumor compound (R)-5b
[0124] Antitumor compound (R)-5b, its structural formula is as follows, and the synthesis steps are as follows:
[0125]
[0126] Preparation of intermediate 8b: Add sodium hydride (60%, 1.5 mmol) to 1-methylpiperidine-3-methanol (1.2 mmol) in N,N-dimethylformamide 10 mL in portions at 0 °C , stirred for 15 minutes, then slowly added intermediate 2 to the mixture at 0°C, slowly warmed the reaction mixture to room temperature and stirred for 1 hour, diluted the reaction solution with water, extracted with ethyl acetate, dried the combined organic layer, and spun Evaporation gave a crude product, which was purified by silica gel column chromatography (dichloromethane:methanol=20:1) to obtain a pale yellow oil, intermediate 8b, yield: 73%. 1 H NMR (400MHz, CDCl 3 )δ8.93(t, J=1.5Hz, 1H), 8.56(dt, J=7.8, 1.3Hz, 1H), 8.47(s, 2H), 8.04(dt, J=7.7, 1.3Hz, 1H), 7.57(t, J=7.8Hz, 1H), 3.97(d, J=5.8Hz, 2H), 2.93(d, J=11.0Hz, 2H), 2.70(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com