Melatonin derivative and preparation method and application thereof
A technology of melatonin and derivatives, applied in the field of melatonin derivatives and its preparation, can solve the problems of limiting research progress, short residence time, low solubility, etc., achieve broad application prospects, prolong residence time, and increase curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
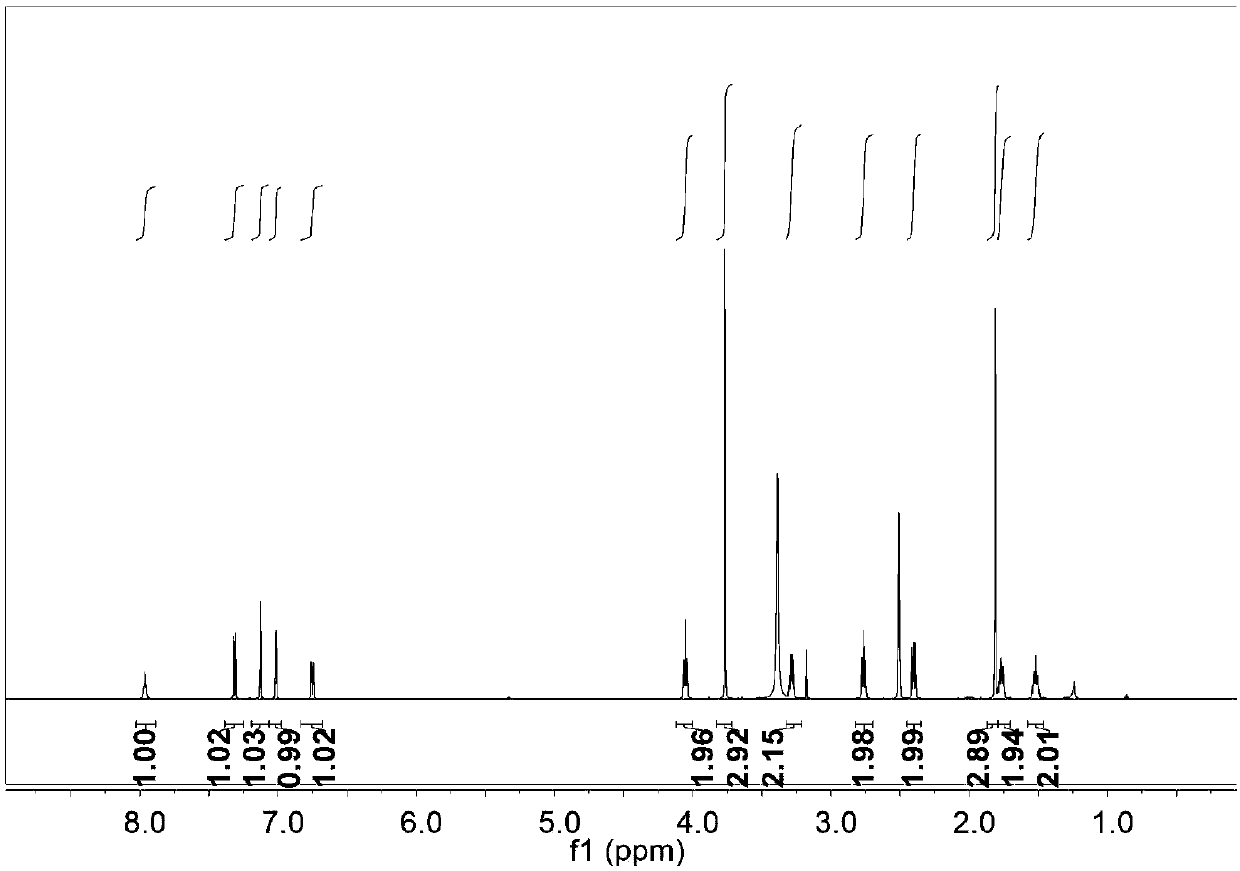
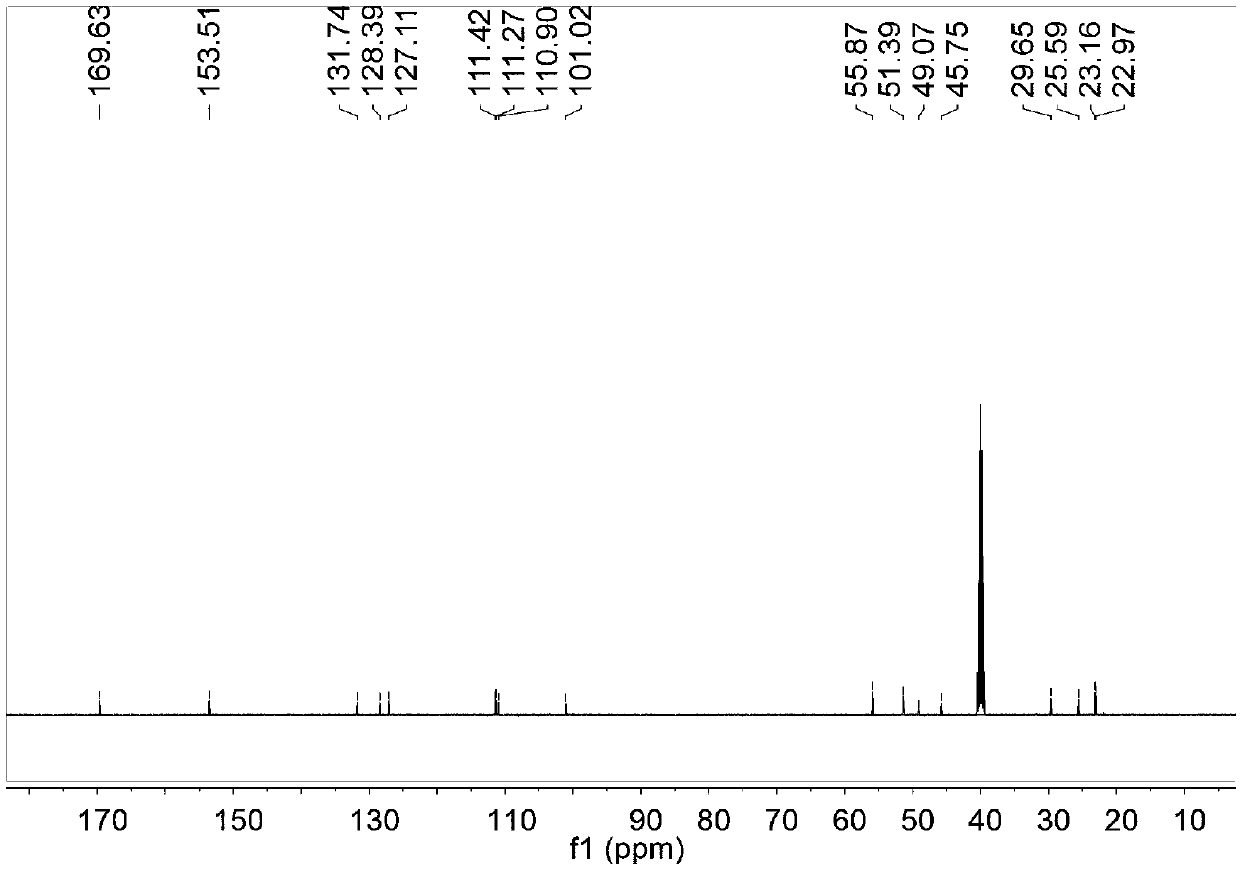
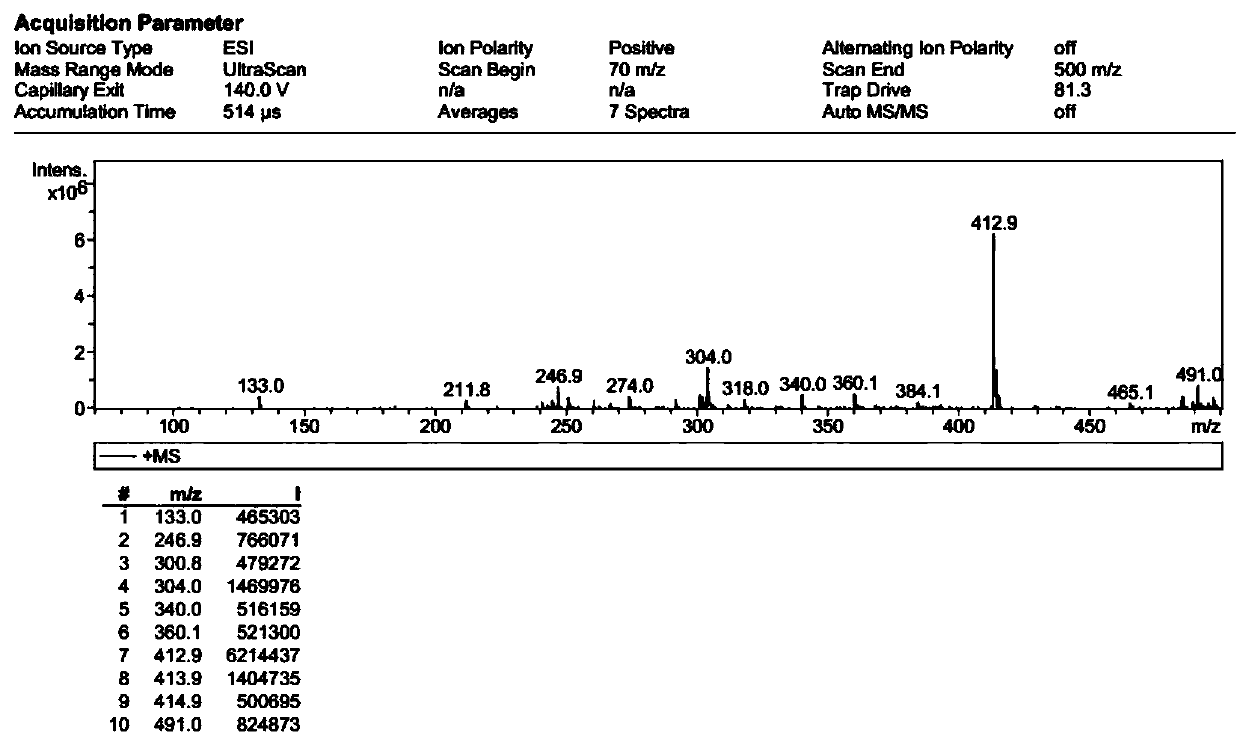
[0037] 100 mg of natural melatonin (Mel) was dissolved in 10 mL of THF (tetrahydrofuran), and then 69 mg of NaH was added on an ice bath. Reacted for 30 minutes while stirring on an ice bath; 88 mg of 1-4 butane sultone was added to the above reaction solution, then the reaction was returned to room temperature, and continued to stir overnight; the reaction was completed by TLC (thin layer chromatography) point plate analysis , add ice water 30mL to the reaction solution to quench the reaction; extract the reaction solution with ethyl acetate, take the water phase, and use a rotary evaporator to remove moisture under reduced pressure; the residue obtained after rotary evaporation is separated and purified by column chromatography to obtain 100mg The target molecular product, Mels, is a light yellow solid, and its hydrogen spectrum, carbon spectrum, and mass spectrum are as follows: Figure 1 to Figure 3 shown. Its synthetic reaction formula is as follows:
[0038]
[0039...
Embodiment 2
[0044]100 mg of melatonin was dissolved in 10 mL of THF (tetrahydrofuran), and then NaH (69 mg) was added on an ice bath. The reaction was stirred for 30 min on an ice bath. 1-3 propane sultone (79 mg) was added to the above reaction solution, then the reaction was returned to room temperature and stirring was continued overnight. The completion of the reaction was analyzed by TLC (thin layer chromatography) spotting, and 30 mL of ice water was added to the reaction liquid to quench the reaction. The reaction solution was extracted with ethyl acetate, the water phase was taken, and the water was removed under reduced pressure with a rotary evaporator. The residue obtained after rotary evaporation was separated and purified by column chromatography to obtain the final target molecular product 1. Its reaction formula is as follows:
[0045]
Embodiment 3
[0047] Dissolve 100 mg of melatonin in 10 mL of acetonitrile, then add 137 mg of sodium carbonate and 136 mg of sodium bromoethylsulfonate, and stir the reaction overnight at 80°C. After the plate monitoring reaction is completed, the residue obtained by spinning and drying is separated and purified by column chromatography to obtain the target molecule 2, and its reaction formula is as follows:
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com