Nickel colloidal catalyst solution for electroless nickel or nickel alloy plating, and method for electroless nickel or nickel alloy plating
A technology of electroless nickel plating and nickel alloy, which is applied in the direction of liquid chemical plating, metal material coating process, coating, etc., can solve the problems such as inability to electroless nickel plating, achieve good appearance uniformity, excellent stability, and prevent mixing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
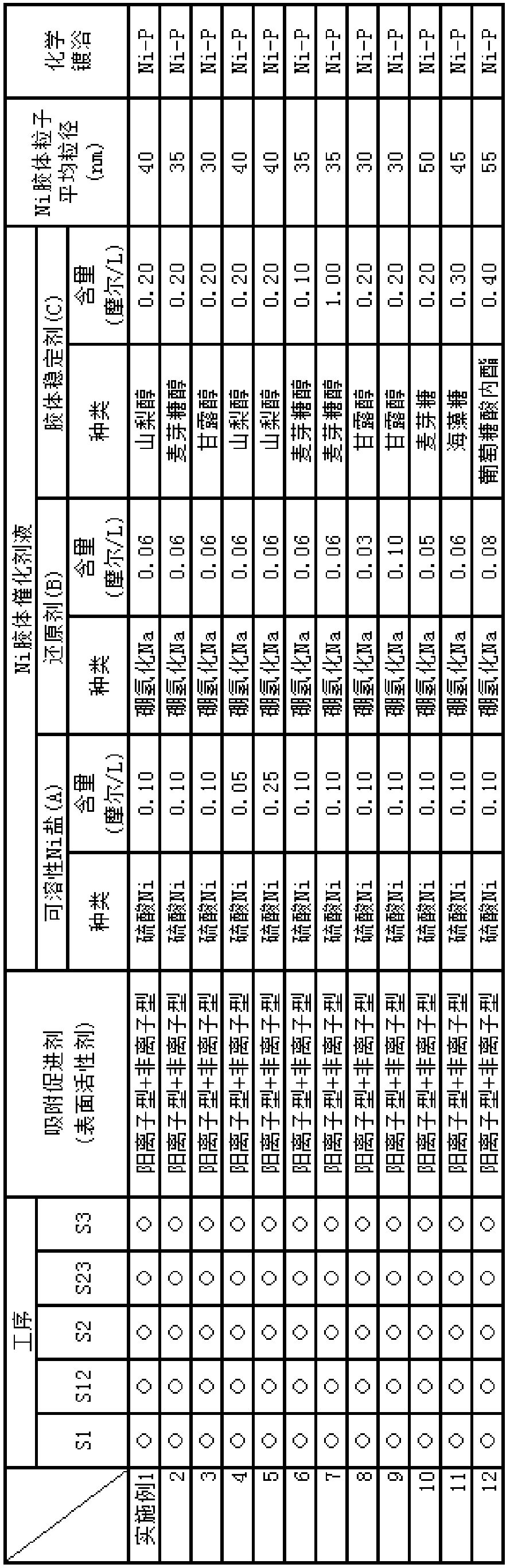
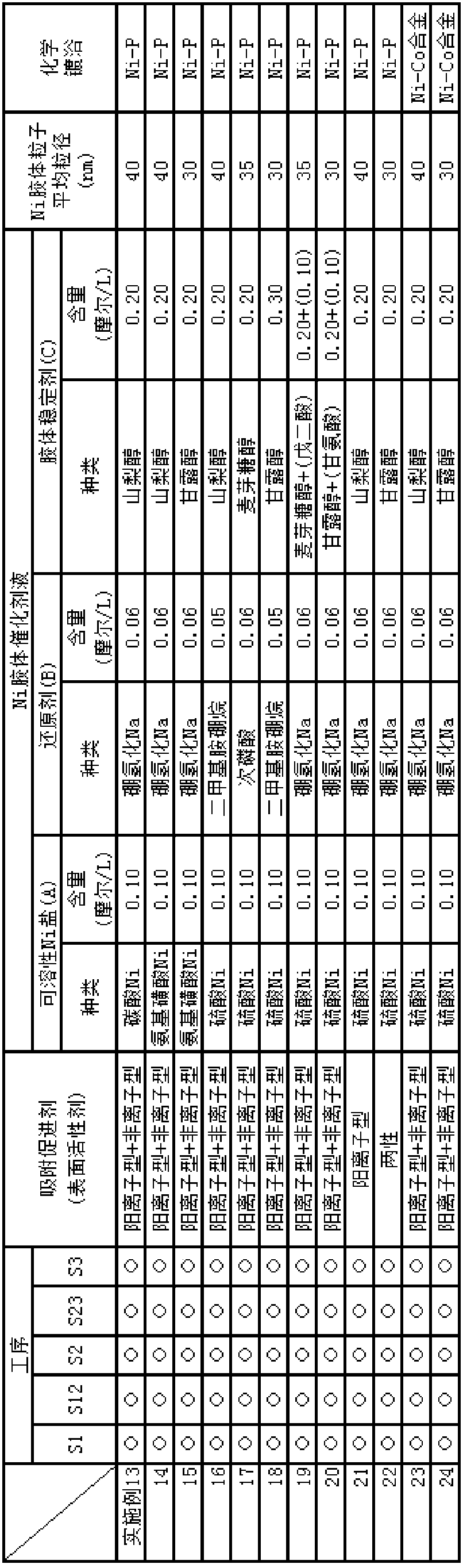
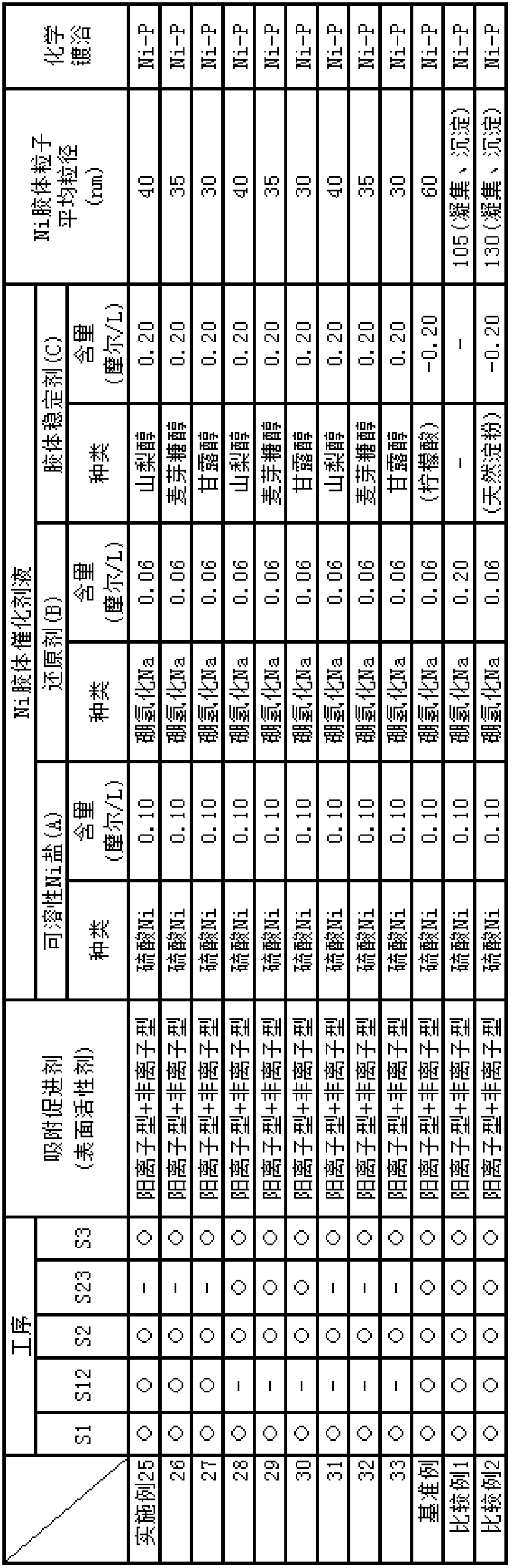
[0141] Below, the embodiment of the electroless nickel plating or the nickel alloy method including the preparation of the liquid containing the adsorption accelerator of the present invention, the nickel colloid catalyst liquid, and the electroless nickel plating or the nickel alloy liquid is described, and it is explained in sequence: preparation Test example of initial nickel colloidal catalyst solution over time, test example of appearance evaluation of nickel (or nickel alloy) film precipitated by electroless plating using the catalyst solution, test example of repeated use resistance when nickel colloidal catalyst solution is used repeatedly An evaluation test example and an appearance evaluation test example of a nickel (or nickel alloy) film deposited by electroless plating using the repeatedly used catalyst solution.
[0142] It should be noted that the present invention is not limited to the following examples and test examples, and it is of course possible to arbitra...
Embodiment 4-5
[0147] Embodiment 4-5: the example of changing the content of soluble nickel salt (A)
Embodiment 10-12
[0148] Embodiment 10-12: change the example of colloid stabilizer (C)
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap