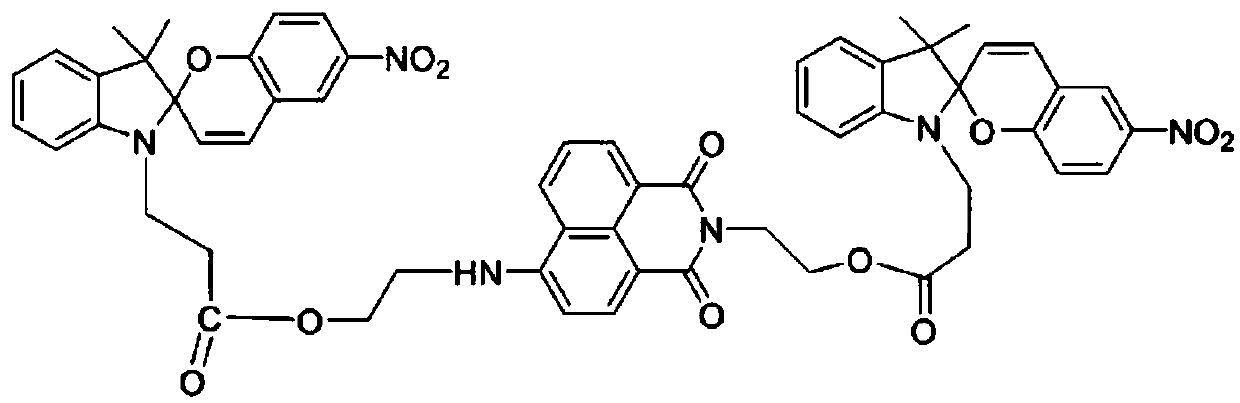
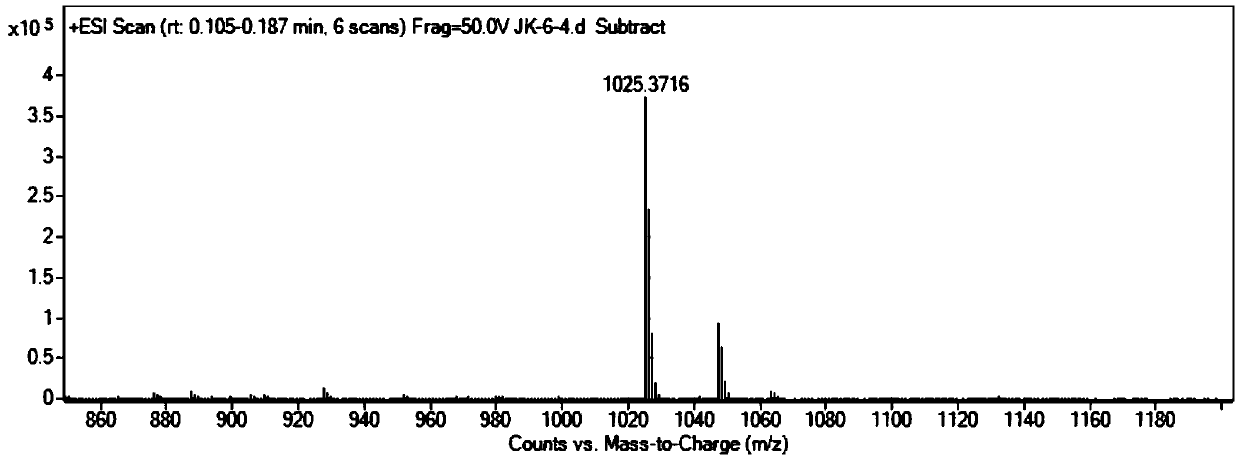
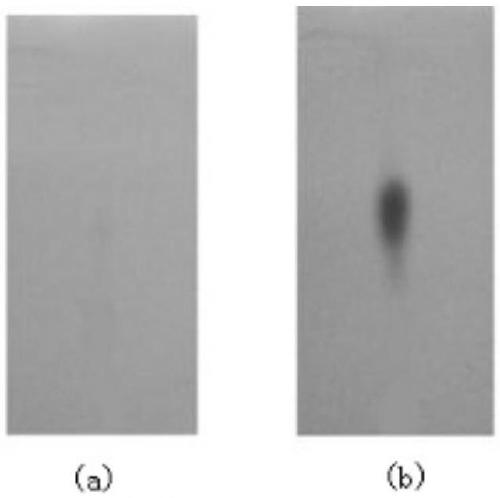
1,8-naphthalimide-based light-controlled fluorescent molecular switch compound bonded with bis-spilenpyran unit and synthesis method and application of compound
A technology of bonding bispiropyran and naphthalimide, which is applied in the fields of chemical instruments and methods, fluorescence/phosphorescence, and material analysis by optical means, can solve the problems of weak photosensitivity and low efficiency of fluorescence fluorescence resonance energy transfer. Advanced problems, to achieve the effect of simple purification method, improved fluorescence resonance energy transfer efficiency, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0043] The concrete synthetic route of present embodiment compound comprises the following steps:
[0044] Synthesis of S1, 2,3,3-trimethylindoline 1
[0045] Dissolve 39.3g of phenylhydrazine hydrochloride in 300mL of glacial acetic acid and heat with stirring. After reflux, add 44mL of methyl isopropyl ketone dropwise. The sodium aqueous solution was neutralized to about pH=7, and extracted three times with ether. The organic layer was dried overnight with anhydrous magnesium sulfate, diethyl ether was distilled off under normal pressure, and the fraction at 80-82°C was collected by distillation under reduced pressure to obtain 10.11 g of compound 1 as a light yellow liquid.
[0046] Synthesis of S2, 3-iodopropionic acid 2
[0047] Under the protection of nitrogen, add 16.65g of anhydrous sodium iodide and 10.84g of 3-chloropropionic acid into a 250mL three-necked flask, then add 100mL of methyl ethyl ketone, heat and reflux for 10 hours with magnetic stirring, filter afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com