Application of ferro-nickel hydrotalcite catalyst in preparation of benzyl alcohol
A technology of benzyl alcohol and catalyst, which is applied in the application field of nickel-iron hydrotalcite catalyst in the preparation of benzyl alcohol, can solve the problems of high production equipment requirements, complicated process, high cost, etc., and achieve reduction of production cost, broaden application range, and facilitate recycling Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The preparation method of the heterogeneous nickel-iron hydrotalcite catalyst used in the present invention, the steps are as follows:
[0042] ①Weigh Ni(NO 3 ) 2 ·6H 2 O and Fe(NO 3 ) 3 9H 2 O and a certain amount of urea are added to the polytetrafluoroethylene-lined hydrothermal reactor, where the molar weight of urea is 3.3 times the sum of the metal molar weight; ② add deionized water to dissolve and magnetically stir at room temperature One hour; ③ After the solid matter is completely dissolved, seal the stainless steel high-pressure reactor, then raise the temperature to 140 ° C, stop heating after 20 hours of hydrothermal reaction, and naturally cool to room temperature; ④ Suction filter the cooled material, and use it separately Wash with ionized water and ethanol for 2 to 5 times, dry in vacuum at 60°C, and grind to obtain a light yellow powder, which is a heterogeneous nickel-iron hydrotalcite catalyst (abbreviated as: Ni-Fe(3 / 1) LDH).
[0043] In addit...
Embodiment 1
[0045] A method for preparing benzyl alcohol using a nickel-iron hydrotalcite catalyst, comprising the following steps:
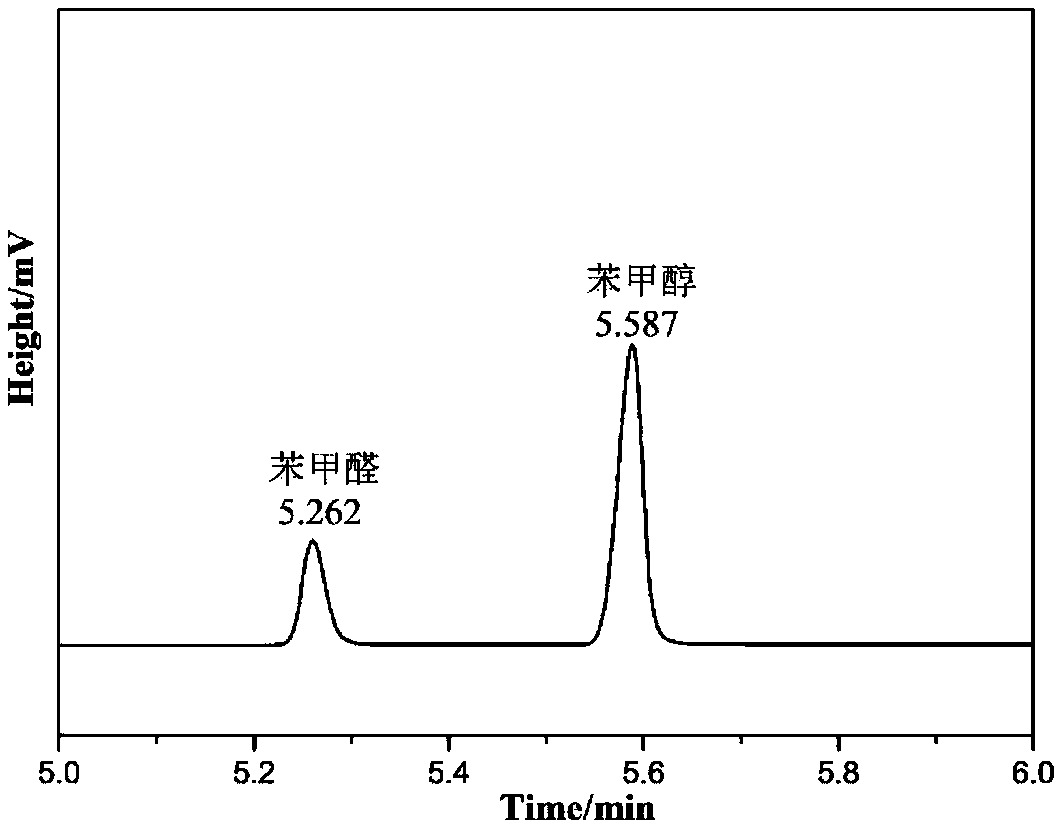
[0046] Add 0.2g of Ni-Fe(3 / 1) LDH to the lining of a clean autoclave, then add 1mmol of benzaldehyde, 5mL of isopropanol and a magnet of appropriate size. After packaging the stainless steel autoclave, Under the action of magnetic stirring, react at 150° C. for 10 h, and after the reaction is completed and cooled, the supernatant is detected and analyzed by a gas chromatograph (such as figure 1 ), it can be seen that benzyl alcohol has been prepared.
Embodiment 2-3
[0048] The present embodiment 2-3 compared the influence of different catalysts on the preparation of benzyl alcohol.
[0049] Add 0.2 g of hydrotalcites (Ni-Fe(2 / 1) LDH and Ni-Fe(4 / 1) LDH) with different nickel-iron molar ratios to the lining of a clean autoclave, and then add 1 mmol of benzaldehyde, 5mL of isopropanol and magnets of appropriate size were packaged in a stainless steel autoclave, and reacted at 150°C for 10 hours under the action of magnetic stirring. After the reaction was completed and cooled, the supernatant was taken for detection and analysis.
[0050]The specific experimental results are shown in Table 1. From the contents of Table 1, it can be seen that when using Ni-Fe(3 / 1) LDH as a catalyst, under the same conditions, the yield and selectivity of benzyl alcohol are the best.
[0051] Catalytic reaction results under different catalysts in table 1
[0052] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com