Hypoglycemic peptide and application thereof
A hypoglycemic and m-aa3 technology, applied in the field of medical biochemistry, can solve problems such as unstable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
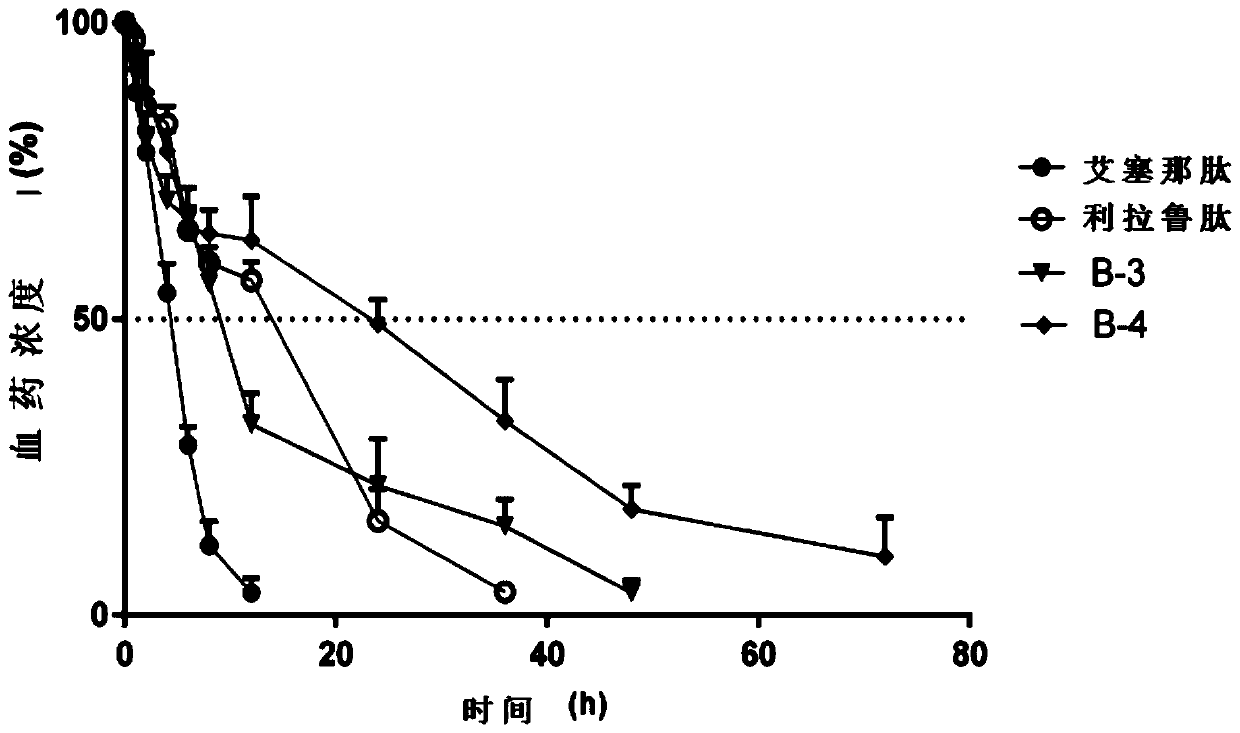
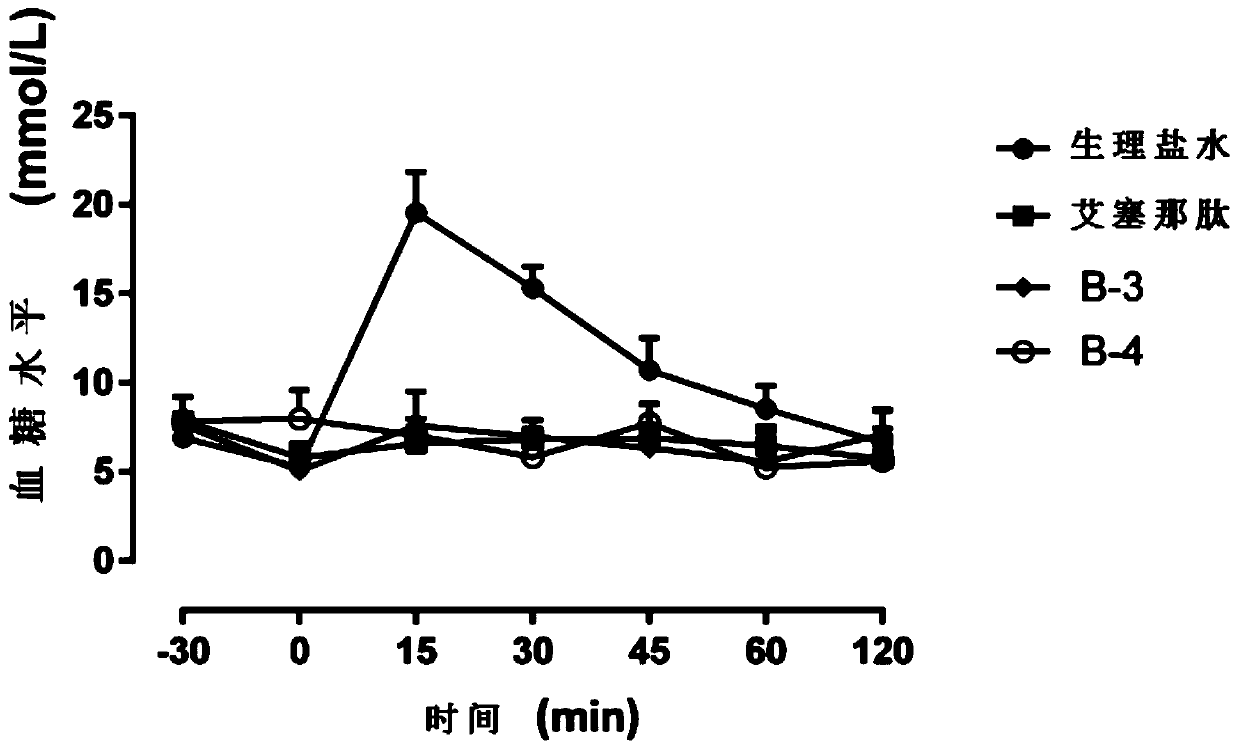
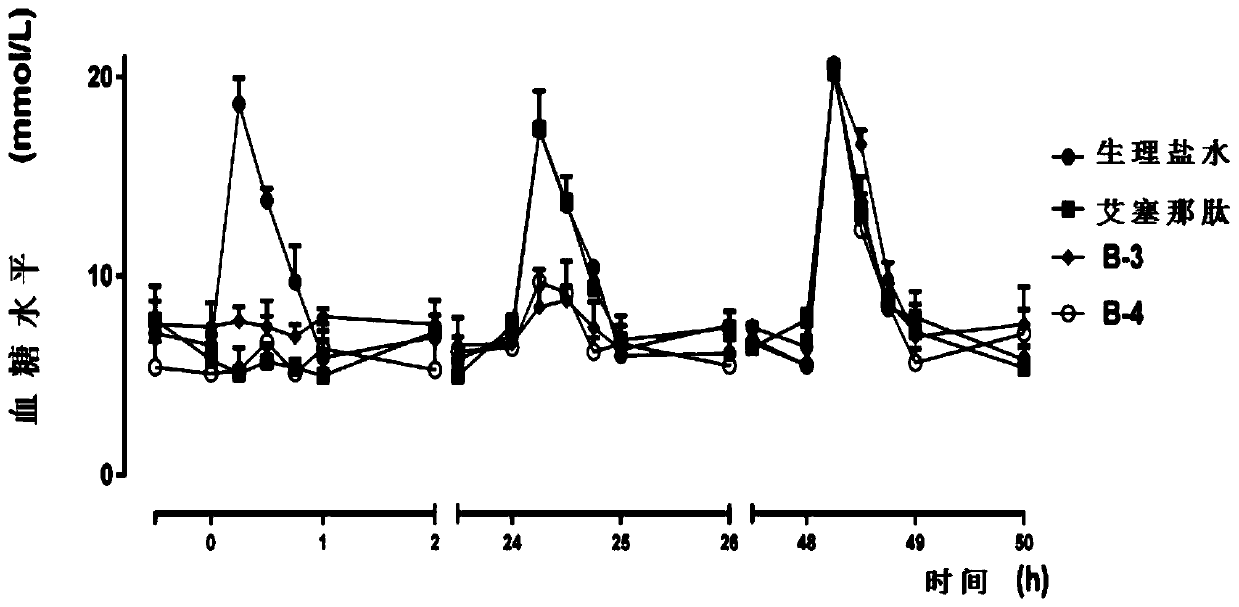
Image
Examples
Embodiment 1
[0089] Example 1: Synthesis of 2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl substituted carboalkanoic acids,
[0090] Method 1.1: Synthesis of 12-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)dodecanoic acid
[0091] Weigh 12-aminododecanoic acid (2151.9 mg) and dissolve it in a three-neck flask with acetic acid, weigh maleic anhydride (980.6 mg) and dissolve it with acetic acid, and slowly add it dropwise to the above solution using a constant pressure dropping funnel , heated to reflux at 120° C. for 6 h, distilled off the solvent under reduced pressure, and purified by basic alumina column chromatography (gradient elution of ethyl acetate / petroleum ether) to obtain a white powdery solid with a yield of 81.9%.
[0092] 1 H-NMR (DMSO-d 6 ,300MHz):δppm:12.42(s,1H,-COO H ),7.50(s,2H,-COC H =C H CO-),3.88(t,2H,J=7.0Hz,-NC H 2 -),2.68(t,J=7.3Hz,2H,-C H 2 COOH),2.00-1.96(m,4H,-NCH 2 C H 2 (CH 2 ) 7 C H 2 ),1.73(s,14H,-NCH 2 CH 2 (C H 2 ) 7 CH 2 ).MS(ESI,m / z):294.1[M-H] - , m...
Embodiment 2
[0097] Embodiment 2: the preparation method of compound X
[0098] The reaction scheme is as follows:
[0099]
[0100] Method 2.1: Synthesis of 12-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)dodecanoyl)-L-glutamic acid
[0101] a) Swelling of 2-CTC resin
[0102]Weigh 2-CTC resin (363.6mg, degree of substitution 0.55mmol / g) into a polypeptide reaction tube, swell with dichloromethane for 30min, wash the resin 3-5 times with dichloromethane, filter and dry for later use.
[0103] b) Synthesis of X-1
[0104] Dissolve Fmoc-Glu(otBu)-OH (255.3mg) and DIPEA (1.6mmol, 264μL) in about 7mL of dichloromethane, pour the solution into the above resin, react at room temperature, bubble nitrogen for 4h, methanol / DIPEA= 9:1 Head sealing for 30 minutes.
[0105] c) Synthesis of X-2
[0106] Wash the above resin three times with DMF and dichloromethane respectively, add about 10 mL of deprotection agent to the reactor, react at room temperature, bubble nitrogen for 30 min, and filter to...
Embodiment 3
[0129] Example 3 Polypeptide Chain Synthesis
[0130] 3.1
[0131] His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Cys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Tyr-Ile-Gln-Trp- Leu-Lys-Glu-Gly-Gly-Pro-Ser-Ser-Gly-Arg-Pro-Pro-Pro-Ser-NH 2
[0132] a) Resin swelling
[0133] Weigh Fmoc-Rink amide-MBHA resin (18.2g, degree of substitution 0.55mmol / g) and place it in a polypeptide reaction tube. After swelling with dichloromethane for 30min, wash it three times with methanol and dichloromethane respectively, and store it for later use.
[0134] b) Removal of 9-hut methoxycarbonyl (Fmoc)
[0135] Preparation of deprotecting agent: Weigh HOBt (6.75g), dissolve it in 400mL of DMF, add 100mL of piperidine, and mix well. Deprotection was performed in two steps. For the first time, add about 50mL of deprotection agent and bubble nitrogen to make the resin fully contact with the deprotection agent. After 30 minutes of deprotection, filter out the deprotection agent, add about 50mL of new de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com