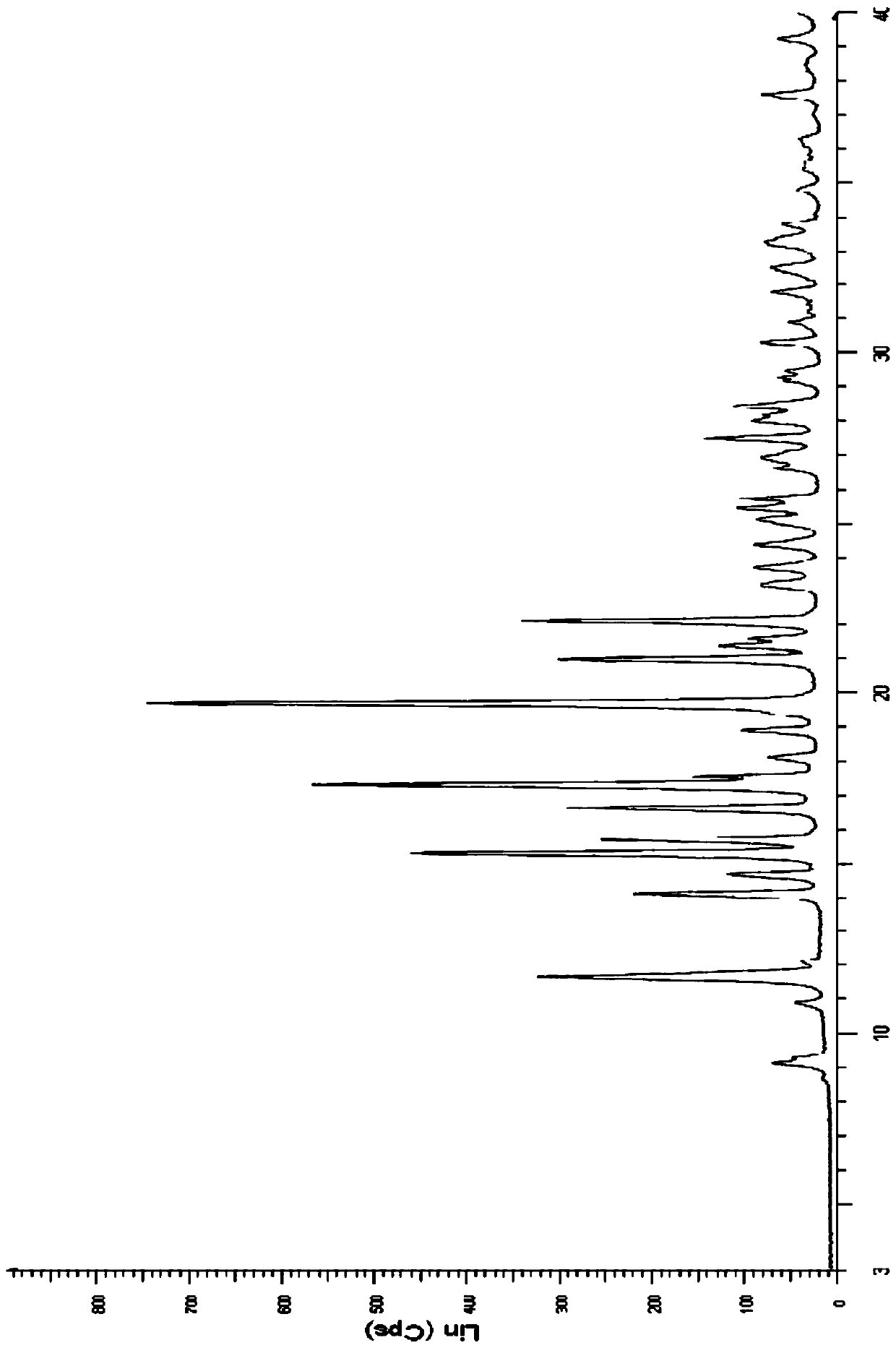
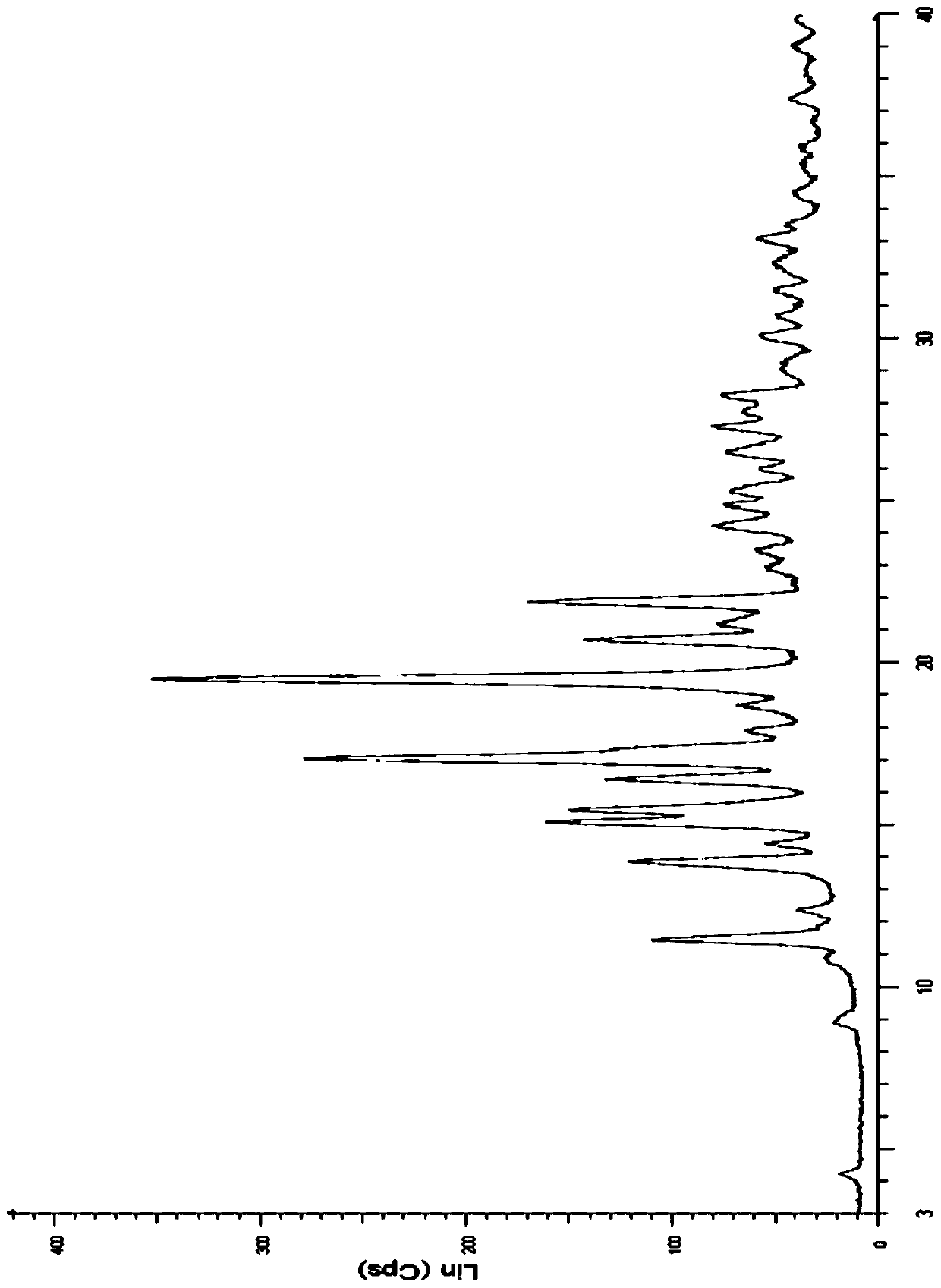
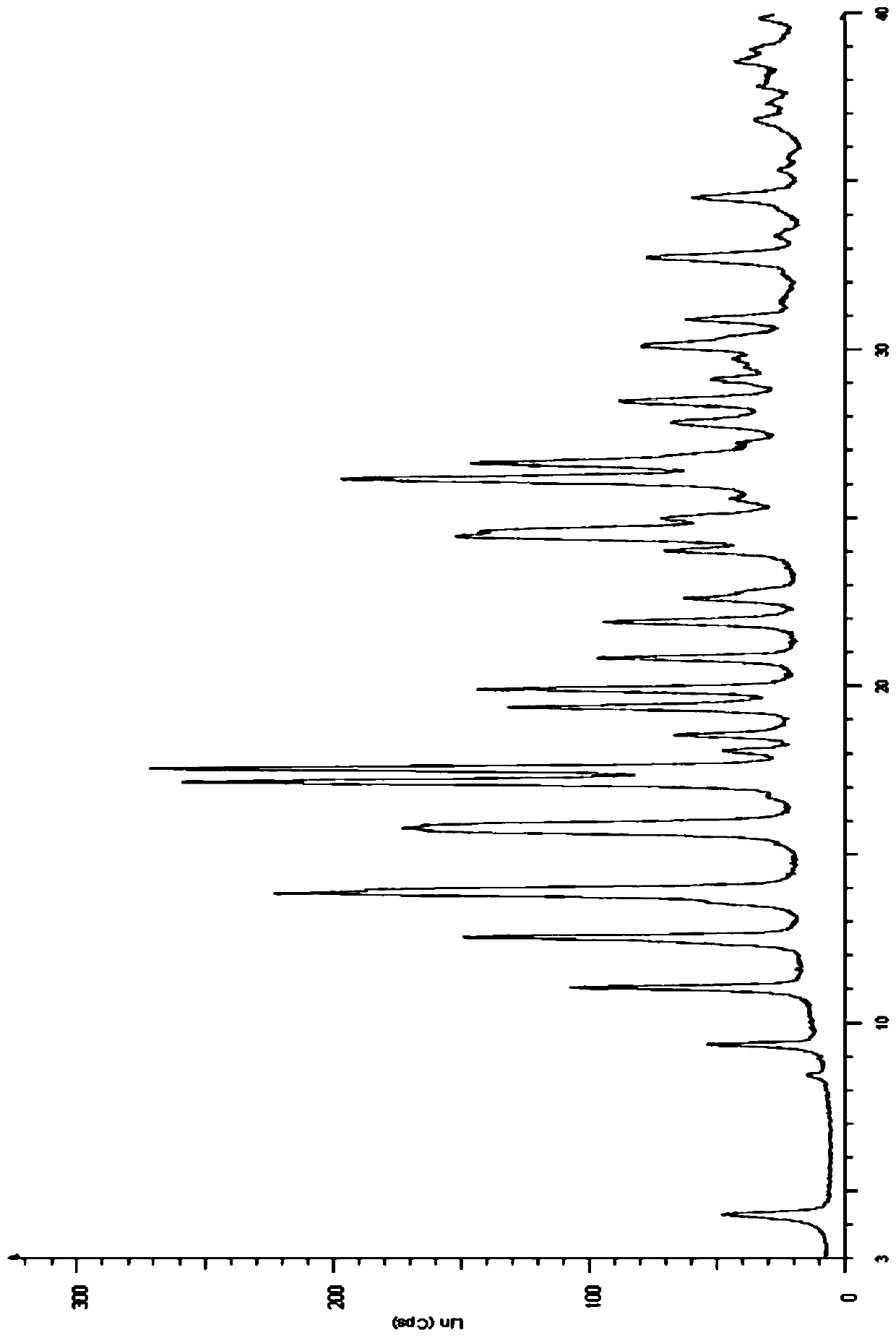
Method for preparing new crystalline form of Lurasidone hydrochloride
A technology of lurasidone hydrochloride and crystal, applied in the field of compounds, can solve the problems of affecting drug absorption and release, affecting drug efficacy and safety, different melting points, solubility and stability, etc. , the effect of fast dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: Preparation of type A lurasidone hydrochloride crystals
[0063] Take 2.50g of lurasidone, dissolve it in about 30ml of ethyl acetate solution, heat at 78°C until completely dissolved, filter while hot, and after cooling down to room temperature, add 1ml of ethyl acetate hydrogen chloride solution drop by drop, continue stirring for 3h, filter, Dry below 50°C to obtain 1.80 g of white solid, which is lurasidone hydrochloride type A crystal.
Embodiment 2
[0064] Embodiment 2: Preparation of type A lurasidone hydrochloride crystals
[0065] Take 2.00g of lurasidone, dissolve it in about 160ml of absolute ethanol solution, heat at 79°C until completely dissolved, filter while hot, and after the solution cools down to room temperature, add 1ml of ethyl acetate hydrogen chloride solution drop by drop, continue stirring for 6h, filter , Dry below 50°C to obtain 1.20 g of white solid, which is lurasidone hydrochloride crystal type A.
Embodiment 3
[0066] Embodiment 3: Preparation of type A lurasidone hydrochloride crystals
[0067] Take 2.00g of lurasidone, dissolve it in about 160ml of isopropanol solution, heat at 83°C until completely dissolved, filter while hot, and after cooling down to room temperature, add 1ml of ethyl acetate hydrogen chloride solution drop by drop, continue stirring for 1h, filter, Dry below 50°C to obtain 1.55 g of white solid, namely type A lurasidone hydrochloride crystals.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com