Novel triterpene compound in pericarpium juglandis as well as preparation method and application thereof
A technology of triterpenoids and compounds, applied in the field of new triterpenoids, which can solve problems such as poor stability, potential safety hazards, and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0029] Embodiment the preparation method of the present invention compound of the first case:
[0030] (1) alcohol extraction: the walnut catalpa Juglans mandshurica The fresh product of Maxim green fruit peel was dried at low temperature at 40°C to obtain 5kg of dry product as raw material, and reflux extracted with 30L of 95% ethanol for 3 times, each time for 2 hours, filtered and combined the ethanol extract, recovered the solvent under reduced pressure, dried, and obtained ethanol-extracted Cream 328g;
[0031] (2) Enrichment and purification: disperse the extract obtained in step (1) with water to a solution with a relative density of 1.18±0.05g / mL, enrich and purify by AB-8 or D101 macroporous resin column chromatography (chromatographic column The specifications are inner diameter × length = 6.5cm × 1.5m, and the effective height of the resin is 1.0m), respectively eluted with water, 50% ethanol, and 80% ethanol in sequence, collected the 80% ethanol eluate, recover...
Embodiment example 2
[0035] Example 2 Structural identification of the compound of the present invention:
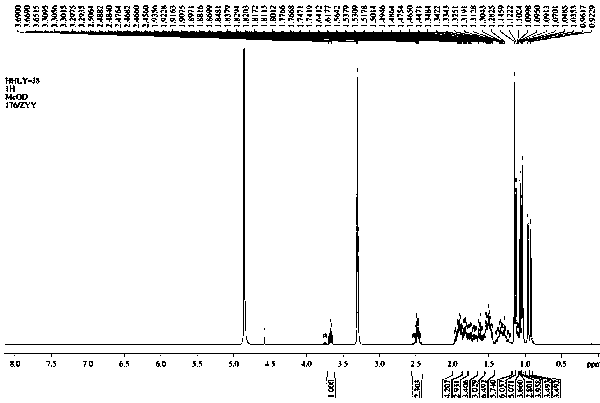
[0036] The compound of the present invention is a white amorphous powder (MeOH). Positive HR-ESI-MS spectra, such as figure 2 shown in m / z Visible at 494.3498[M] + The ion peak indicates that the molecular weight of the compound is 494. combine 1 H-NMR, 13 C-NMR and DEPT spectra speculate that its molecular formula is C 33 h 50 o 3 , calculate its degree of unsaturation to be 9.
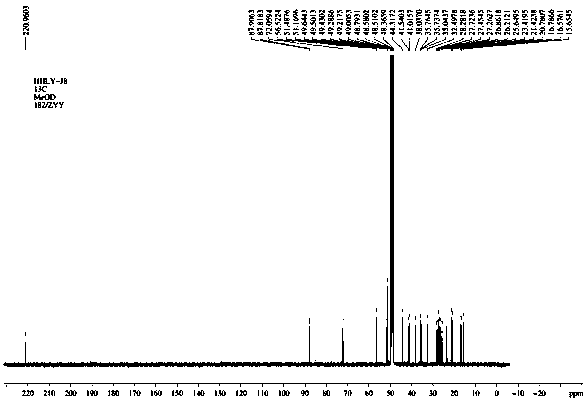
[0037] in the compound 1 H-NMR (Methanol- d 4 , 400MHz) spectrum, such as figure 1 , showing that the 8 methyl protons are δH 0.92 (H-26), 0.96 (H-26), 1.04 (H-30), 1.05 (H-27), 1.07 (H-29), 1.12 (H-24), 1.15 (H-28), and 1.15 (H-23), an oxygen-linked methine proton signals at δ H 3.67 (H-3). 13 C-NMR (Methanol- d 4 , 100MHz) spectrum combined with DEPT spectrum, such as Figure 4 , Figure 5 As shown, there are 33 carbon signals, including 8 methyl groups, 10 methylene groups, 5 methin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com