C-peptide immunogen, monoclonal antibody pair thereof, and application of monoclonal antibody pair to C-peptide magnetic particle chemical light-emitting immunoreagent
A monoclonal antibody and peptide immunogen technology, applied in the field of immunodiagnosis, can solve the problems of complicated operation, affecting the application of diagnostic reagents, low immunogenicity of C-peptide, etc., and achieve the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Polypeptide synthesis
[0037] Synthesize the full-length sequence of C peptide (1-31aa)EAEDLQVGQVELGGGPGAGSLQPLALEGSLQC (SEQ IDNO:17), N-terminal amino acid sequence (1-12aa)EAEDLQVGQVELGGC, C-terminal amino acid sequence (18-31aa)CGGAGSLQPLALEGSLQ, N-terminal amino acid sequence or C-terminal amino acid GGC or CGG in the sequence serves as a connecting arm and a sulfhydryl group when coupled with a carrier protein. In addition, synthesized short peptides EAEDLQVGQVELGGGPG(1-17aa), EAEDLQVGQVELGG(1-14aa), EAEDLQVGQVEL(1-12aa), EAEDLQVGQV(1-10aa), EDLQVGQVELGGGPG(3-17aa), LQVGQVELGGGPG(5-7aa), VG7VELGGGPG( -17aa), GGPGAGSLQPLALEGSLQ (14-31aa), GGPGAGSLQPLALEGS(14-29aa), GGPGAGSLQPLALE(1-27aa), GGPGAGSLQPLA(14-25aa), PGAGSLQPLALEGSLQ(16-31aa), 13AGSLQPLALEGSLQ (18-31aaPLAL0PLALEG), 14QSLQ( 31aa).
[0038] 2. Synthesis of the first complete antigen and the second complete antigen
[0039] Weigh 3mg of BSA and dissolve it in 600ul of PBS pH7.4; weigh 0.5mg of sulf-SM...
Embodiment 2
[0041] 1. Animal immunity
[0042] Strong female Balb / c mice aged 6-8 weeks were selected for immunization. For the first immunization, Freund's complete adjuvant was used, 100 μg / mouse, multi-point injection in the armpit; for booster immunization, Freund's incomplete adjuvant was used, 50 μg / mouse, 3 times of booster immunization, and the interval between adjacent times of booster immunization 2 weeks; the interval between the first immunization and the first booster immunization is 3 weeks. Eyeball blood was collected 1 week after the last booster immunization, and the full-length C-peptide antigen was coated on a microtiter plate, and the antiserum titer was determined by indirect ELISA. 3 days before fusion, 100 μg antigen / mouse intraperitoneal injection
[0043] 2. Cell fusion and monoclonal antibody preparation
[0044] Mice that had been impacted in advance were killed by neck dislocation, and sterilized by soaking in 75% ethanol. In the ultra-clean workbench, the ...
Embodiment 3
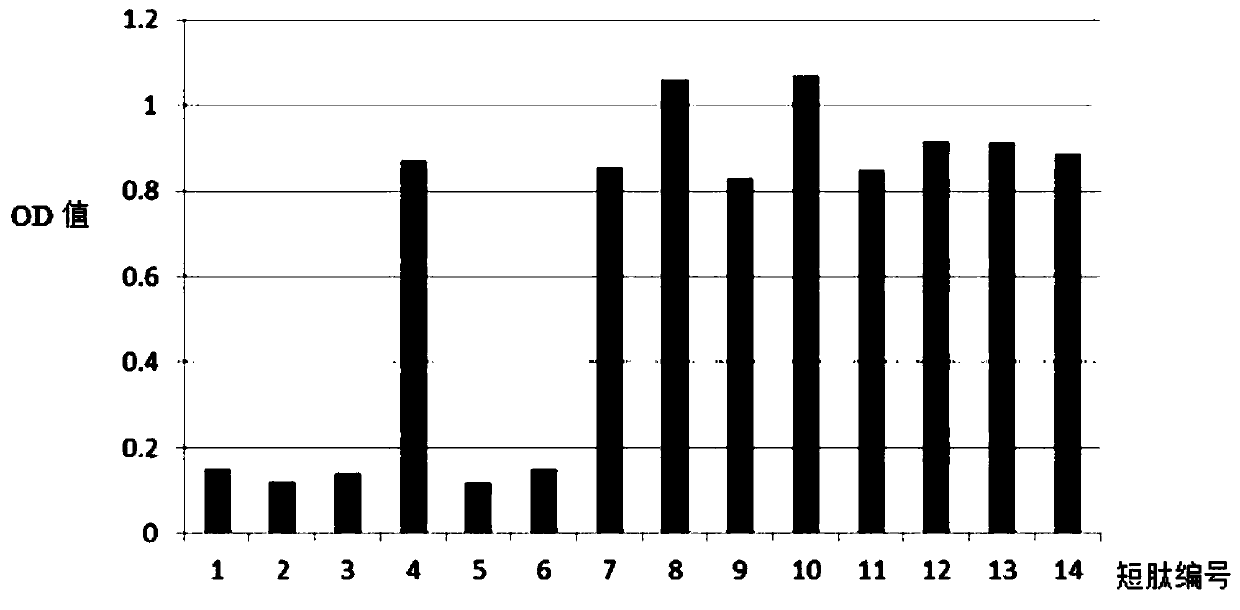
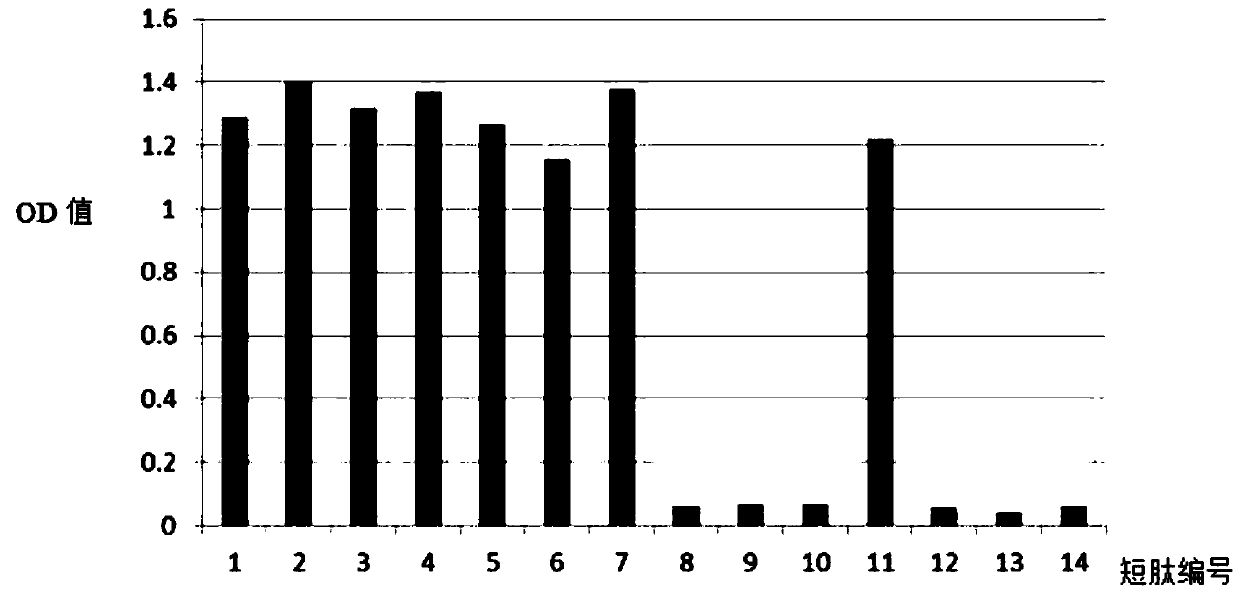
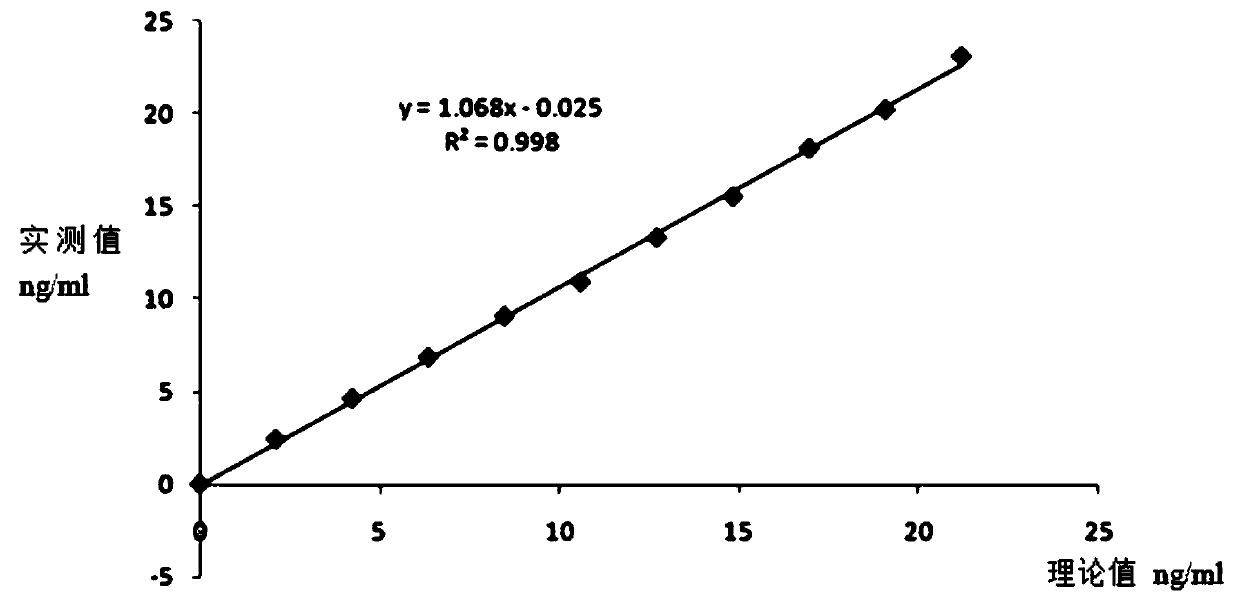
[0047] The full-length C-peptide antigen was coated on a 96-well plate with a coating concentration of 2ug / ml, 4°C for 2 hours; 1% casein was blocked at 37°C for 1 small test, and washed three times with PBS pH7.4; the synthetic short peptide SEQ ID NO: 3 to SEQ ID NO: 16 were diluted to 15ug / ml, and added to 50ul / well; monoclonal antibody 50ul / well, 1ug / ml, reacted at 37°C for 1 hour, washed three times with PBS pH7.4; added 1:2000 Dilute goat anti-mouse secondary antibody, 100ul / well, react at 37°C for 1 hour, wash with PBS pH7.4 four times; develop color for 5 minutes, and terminate the reaction. After reading and analyzing the data, the consensus sequence of all short peptide sequences that can compete for the reaction is the binding site of the monoclonal antibody.
[0048] Such as figure 1 As shown, the N-terminal monoclonal antibody can react with short peptides SEQ ID NO: 3 to 5 and SEQ ID NO: 7 to 8, and the 5-12 amino acids of the consensus sequence are the N-termi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com