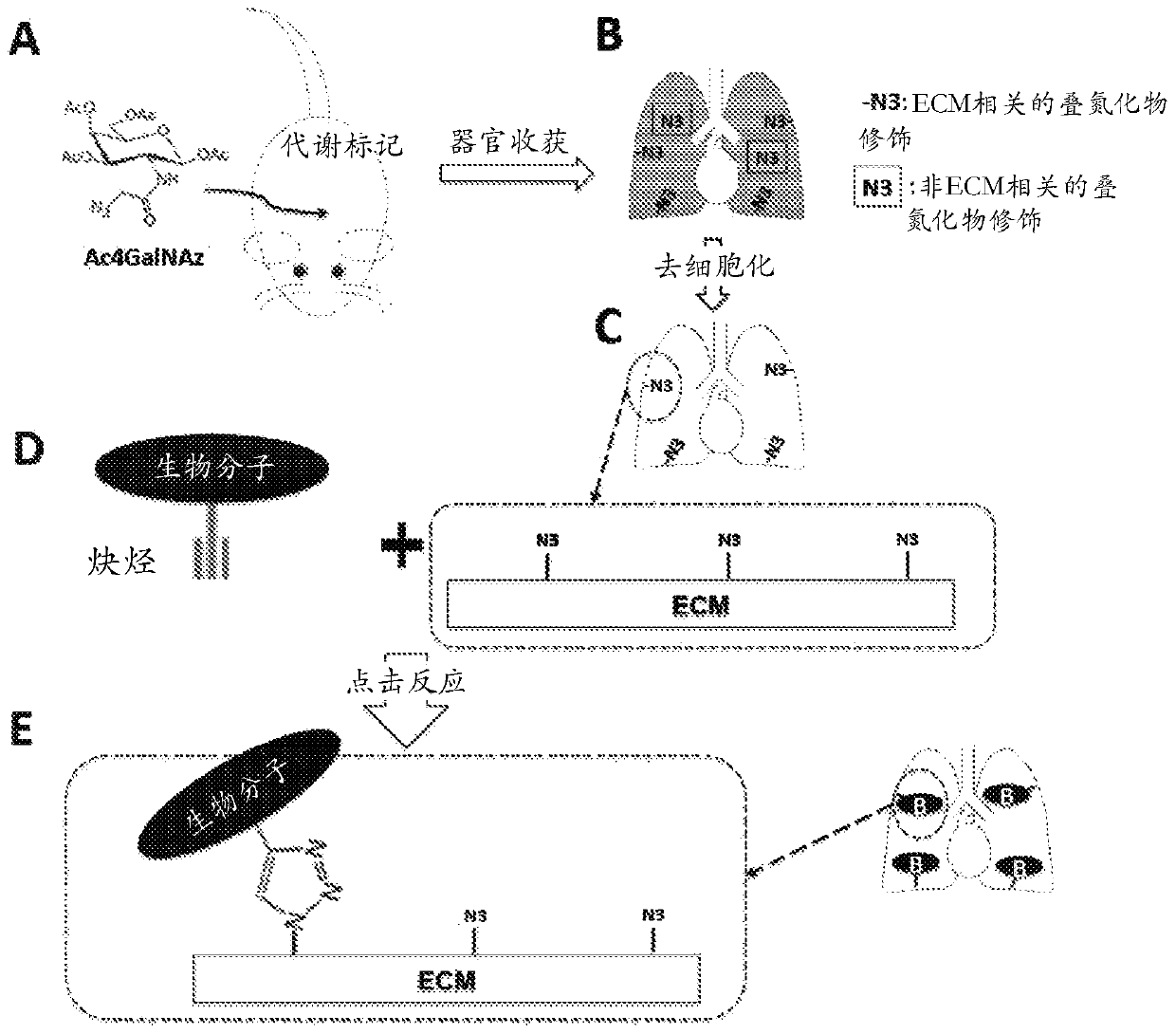
Metabolic labeling and molecular enhancement of biological materials using bioorthogonal reactions
A bioorthogonal, bioactive molecular technology for metabolic labeling and molecular enhancement of biomaterials using bioorthogonal reactions to address issues affecting short- and long-term graft outcomes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0357] The preparation of the compounds provided herein may involve the protection and deprotection of various chemical groups. The need for protection and deprotection and selection of appropriate protecting groups can be readily determined by those skilled in the art. The chemical properties of protecting groups can be found, for example, in P.G.M. Wuts and T.W. Greene, Protective Groups in Organic Synthesis [Protective Groups in Organic Synthesis], 4th Edition, Wiley & Sons, Inc. [Wiley & Sons], New York (2006) turn up.
[0358] In some embodiments, growth factors such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) can be obtained by using alkyne-containing or alkyne-containing N-hydroxysuccinimide esters (NHS esters). Cycloalkyne reagents are conjugated to alkyne or cycloalkyne (eg, DBCO) functional groups. A PEG linker or an alkylene linker, or both, of various lengths can be introduced between the alkyne or cycloalkyne functional...
example
[0503] Materials and General Methods
[0504] In vivo metabolic engineering and organ / tissue decellularization
[0505] All animal experiments were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and performed in accordance with the Animal Welfare Act. Male Sprague-Dawley rats (100-125 g, Charles River Laboratories) were given metabolic labeling reagents (Ac4GalNAz, Ac4GlcNAz or Ac4ManNAz) (30 mg / day, Click Chemistry Tools) daily via intraperitoneal injection for three days. One day after the last administration of metabolic labeling reagents, organs were harvested from animals and decellularized by perfusion using the following conditions: 0.1% SDS through the pulmonary artery (PA) for the lungs; 1% SDS retrograde coronary perfusion through the ascending aorta for the heart 1% SDS is delivered to the kidneys via the renal artery; and 1% SDS is delivered to the liver via the inferior vena cava (where the superior vena cava is ligated...
example 1- 3
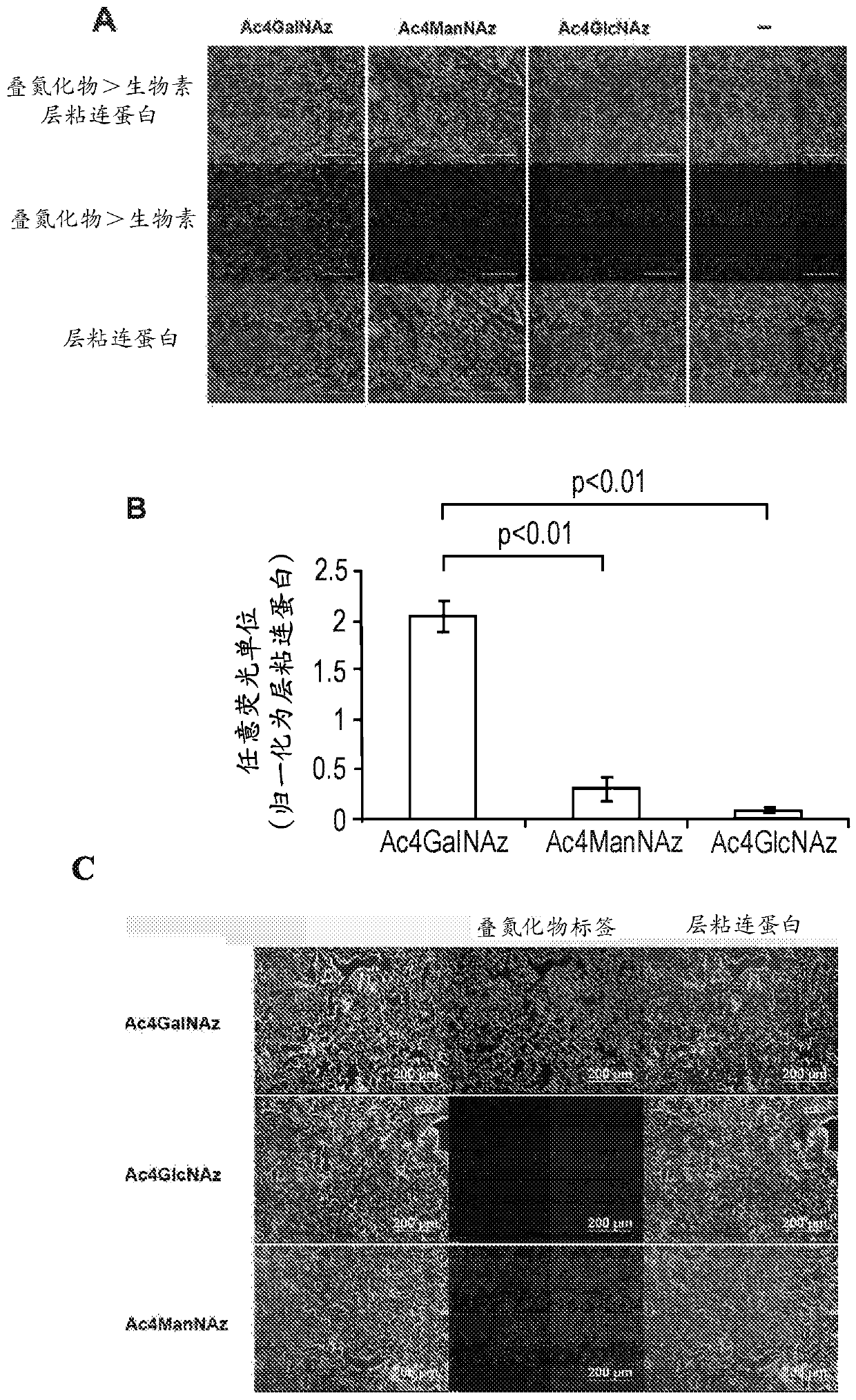
[0520] Example 1 - Comparison of metabolic labeling efficiencies of three azide-labeled sugars (Ac4GalNAz, Ac4GlcNAz and Ac4ManNAz)
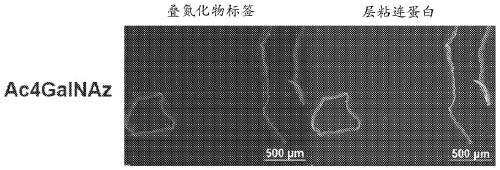
[0521] The first step in the presently described method and procedure is to generate ligands (azide tags) on decellularized organ / tissue scaffolds by metabolic labeling using azide-tagged sugars for chemoselective ligation (click reaction). ). In the method described, azide-labeled galactosamine (Ac4GalNAz) is used to metabolically label decellularized native organ / tissue scaffolds. Using decellularized lung scaffolds as a model, it was demonstrated that Ac4GalNAz exhibited higher labeling efficiency compared to other commercially available azide-labeled sugars such as Ac4GlcNAz and Ac4ManNAz.
[0522] exist figure 2 In A, images showing staining for azide labeling (purple) and the ECM component laminin (green) on decellularized rat lungs after 3 days of metabolic labeling in donor rats. Using biotin-alkyne (via click reaction) (Sigma-Aldri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com