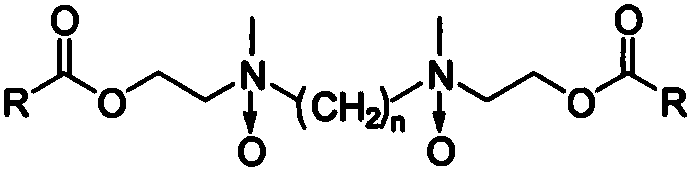
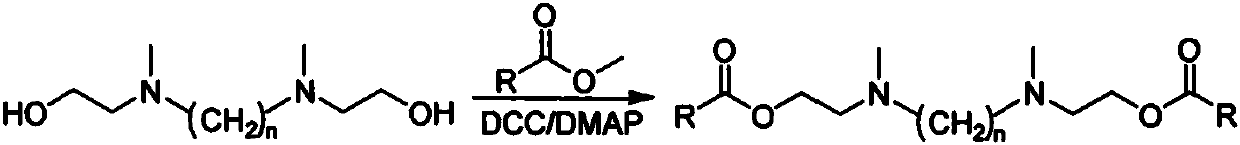
Preparation method of gemini amine oxide containing alkyl group, ester group and ethyl group
A technology of alkyl ester and amine oxide, applied in organic chemistry and other directions, can solve the problems of uneasy control of reaction conditions, unenvironmental protection, residues, etc., and achieve the effects of convenient production process, low irritation and mild synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Preparation of N,N'-dimethyl-bis(2-hydroxyethyl)-ethylenediamine
[0018] Add 0.1mol N,N'-bis(2-hydroxyethyl)-ethylenediamine and 30mL methanol into a 250mL three-necked flask, slowly add 0.7mol formic acid dropwise while stirring, and then slowly dropwise add 0.6mol formaldehyde aqueous solution, and heat to 70 ℃, constant temperature reaction 4h. After the reaction is completed, cool to room temperature, add NaOH to fully dissolve the solid, adjust the pH value to 10-12, precipitate a white solid, filter with suction, and dry to obtain N,N'-dimethyl-bis(2-hydroxyethyl)- Ethylenediamine.
[0019] (2) Preparation of N,N'-dimethyl-bis(2-lauryl ester ethyl)-ethylenediamine
[0020] Add 0.1mol N,N'-dimethyl-bis(2-hydroxyethyl)-ethylenediamine, 0.3mol lauric acid and 30mL dichloromethane into a 250mL round-bottom flask. After stirring for 10min at room temperature, add 0.3mol bicyclic Hexylcarbodiimide and 0.5wt% 4-dimethylaminopyridine as the reaction substrate are used as...
Embodiment 2
[0024] (1) Preparation of N,N'-dimethyl-bis(2-hydroxyethyl)-ethylenediamine
[0025] Add 0.1mol N,N'-bis(2-hydroxyethyl)-ethylenediamine and 30mL methanol into a 250mL three-necked flask, slowly add 0.7mol formic acid dropwise while stirring, and then slowly dropwise add 0.6mol formaldehyde aqueous solution, and heat to 70 ℃, constant temperature reaction 4h. After the reaction is completed, cool to room temperature, add NaOH to fully dissolve the solid, adjust the pH value to 10-12, precipitate a white solid, filter with suction, and dry to obtain N,N'-dimethyl-bis(2-hydroxyethyl)- Ethylenediamine.
[0026] (2) Preparation of N,N'-dimethyl-bis(2-myristyl ester ethyl)-ethylenediamine
[0027] Add 0.1mol N,N'-dimethyl-bis(2-hydroxyethyl)-ethylenediamine, 0.3mol myristic acid and 30mL dichloromethane into a 250mL round-bottomed flask. After stirring for 10min at room temperature, add 0.3mol Cyclohexylcarbodiimide and 0.5wt% 4-dimethylaminopyridine as a reaction substrate are used as...
Embodiment 3
[0031] (1) Preparation of N,N'-dimethyl-bis(2-hydroxyethyl)-1,3-propanediamine
[0032] Add 0.1mol N,N′-bis(2-hydroxyethyl)-1,3-propanediamine and 30mL methanol into a 250mL three-necked flask, slowly add 0.7mol formic acid dropwise while stirring, and then slowly dropwise add 0.6mol formaldehyde aqueous solution. , Heat to 70°C and react at constant temperature for 4h. After the reaction is completed, cool to room temperature, add NaOH to fully dissolve the solid, adjust the pH value to 10-12, precipitate a white solid, filter with suction, and dry to obtain N,N'-dimethyl-bis(2-hydroxyethyl)- 1,3-propanediamine.
[0033] (2) Preparation of N,N'-dimethyl-bis(2-palmityl ester ethyl)-1,3-propanediamine
[0034] Add 0.1mol N,N'-dimethyl-bis(2-hydroxyethyl)-1,3-propanediamine, 0.3mol palmitic acid and 30mL dichloromethane into a 250mL round bottom flask. After stirring for 10min at room temperature, add 0.3 mol of dicyclohexylcarbodiimide and 0.5 wt% of 4-dimethylaminopyridine as a re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com