Oral cavity instant membrane agent containing rizatriptan benzoate and preparation method thereof
A technology of rizatriptan benzoate and oral instant film, which is applied in the field of medicine, can solve problems such as rising, drug recrystallization, and uneven drug distribution, and achieve simple process, shortened production cycle, and stable drug production quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
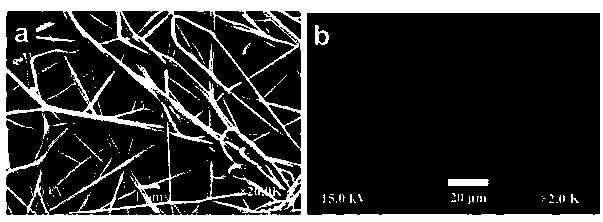
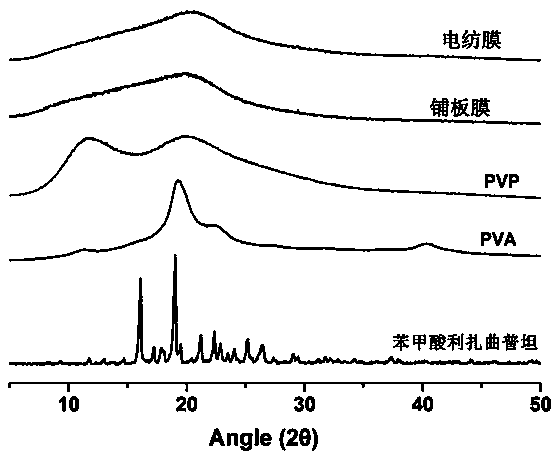
Image
Examples
Embodiment 1
[0042] prescription:
[0043] Rizatriptan Benzoate 363.25mg
[0044] Polyvinyl alcohol 18-88 1.651g
[0045] Preparation method: dissolve polyvinyl alcohol 18-88 in 30ml of water, add rizatriptan benzoate, stir to dissolve it, and let stand for 3 hours to remove air bubbles. Inhale the above mixed solution into the syringe, control the liquid flow to 0.6ml / h, the electrospinning voltage to 15kV, and the receiving distance to 15cm, and wrap the receiving plate with aluminum foil to start collecting the nanofiber membrane. The collected nanofibrous membranes were dried in a vacuum desiccator for 3 days. Then remove the film and cut into films containing rizatriptan 5 / 10mg each.
Embodiment 2
[0047] prescription:
[0048] Rizatriptan Benzoate 363mg
[0049] Polyvinylpyrrolidone K60 1.65g
[0050] Preparation method: Dissolve polyvinylpyrrolidone K60 in 35ml of water, add rizatriptan benzoate, stir to dissolve, and let stand for 3 hours to remove air bubbles. Inhale the above mixed solution into the syringe, control the flow rate to 0.6ml / h, the electrospinning voltage is 16kV, the receiving distance is 15cm, wrap the receiving plate with aluminum foil, and start collecting the nanofiber membrane. The obtained nanofibrous film is placed in a vacuum desiccator and dried for 3 days, and the film is removed and cut into films containing 5 / 10 mg of rizatriptan.
Embodiment 3
[0052] prescription:
[0053] Rizatriptan Benzoate 363.25mg
[0054] Polyoxyethylene alcohol N-80 1.65g
[0055] Preparation method: dissolve polyoxyethylene N-80 in 30ml of water, add rizatriptan benzoate, stir to dissolve it, and let stand for 3 hours to remove air bubbles. Inhale the above mixed solution into the syringe, control the flow rate of 0.6 ml / h, the voltage of electrospinning is 18kV, the receiving distance is 16cm, the receiving plate is wrapped with aluminum foil, and the nanofiber membrane is collected. The nanofibrous membrane that collects is placed in vacuum desiccator and dried for 3 days, defilmed, and cut into each film containing rizatriptan 5 / 10mg to get final product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com