Method for preparing nitrogen doped titanium dioxide with high-energy crystal surface on surface
A titanium dioxide and nitrogen doping technology, which is applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problem of low electron-hole pair separation efficiency, narrow light absorption range, and unsatisfactory effects, etc. problems, to achieve the effect of active chemical properties, good repeatability and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
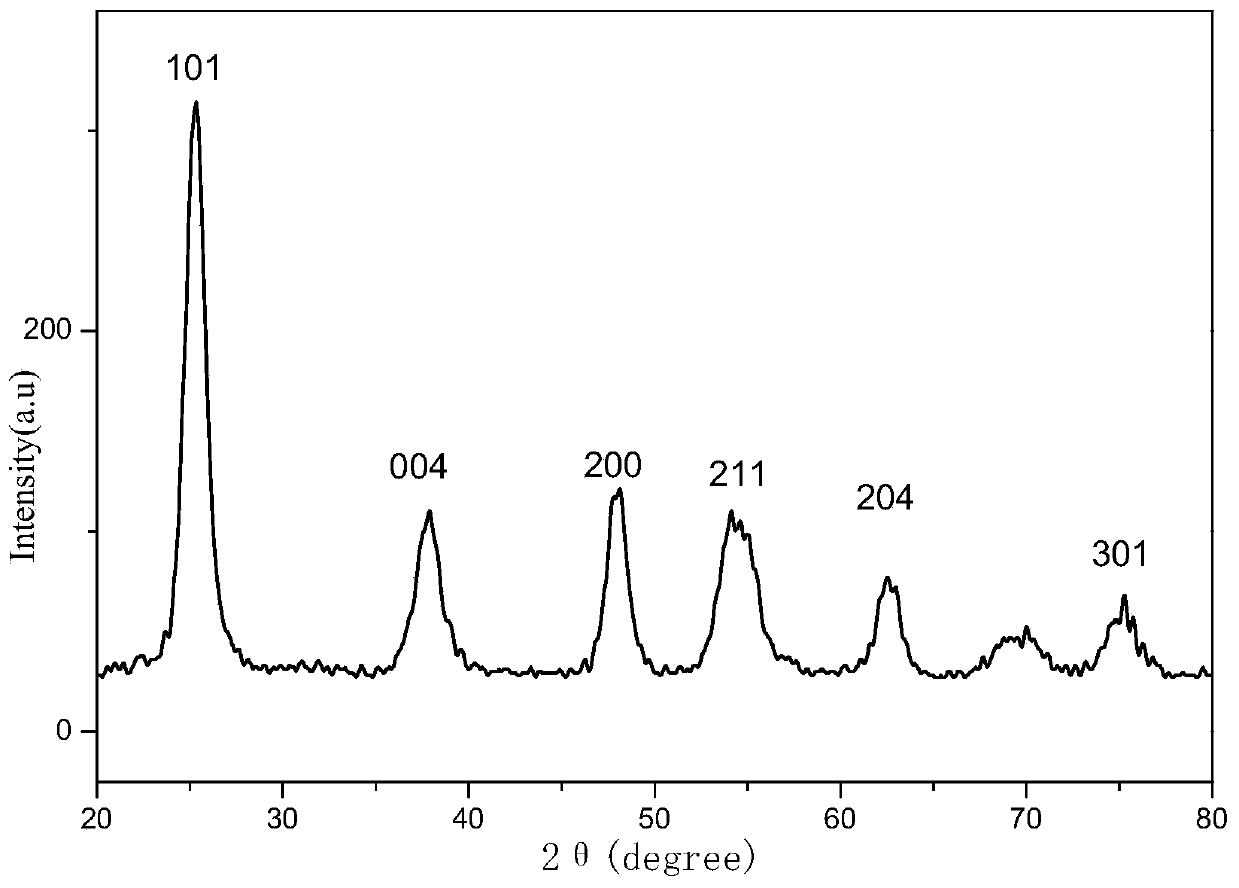
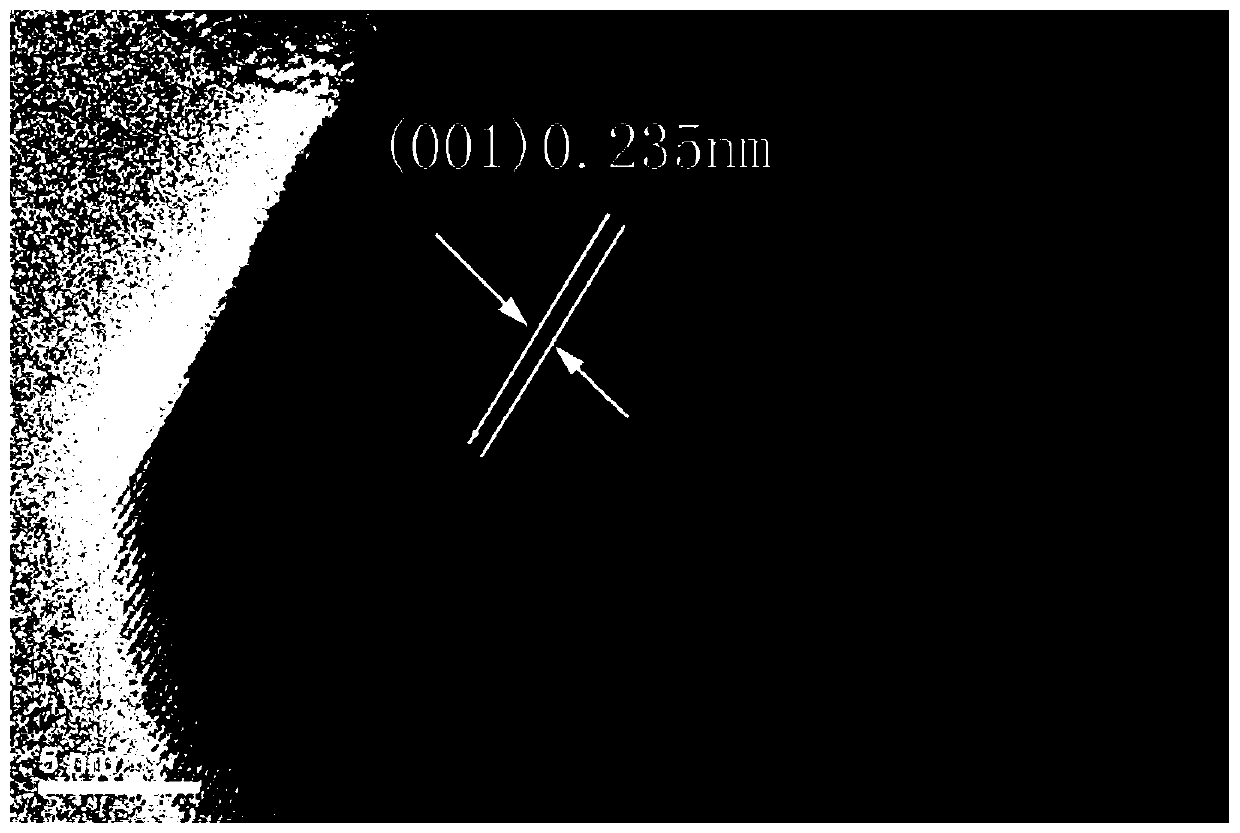
Image
Examples
Embodiment 1
[0035] Accurately add 50ml of ethanol to a 100ml beaker, then weigh 1mmol of urea and dissolve it in ethanol, place the beaker on a magnetic stirrer and stir continuously, add 3mmol of titanium tetrachloride dropwise in a fume hood, and wait until the titanium tetrachloride solution is completely After dispersion in ethanol, a pale yellow solution was obtained at this point.
[0036] Then add 3mmol titanium tetrafluoride to the beaker, stir well for 15 minutes, transfer the resulting suspension into a 100ml autoclave, and react at 180°C for 12 hours. After cooling to room temperature, use 40ml of the obtained solid powder Wash 3 times with absolute ethanol, then wash 3 times with 40ml distilled water, and then wash 2 times with 40ml absolute ethanol. A khaki solid powder was finally obtained, which was then collected by centrifugation, dried at 60°C for 12 hours, and the dried product was taken out, then placed in a muffle furnace and roasted at 200°C for 1 hour, and then grou...
Embodiment 2
[0042] Accurately add 50ml of ethanol to a 100ml beaker, then weigh 6mmol of urea and dissolve it in ethanol, place the beaker on a magnetic stirrer and stir continuously, add 3mmol of titanium tetrachloride dropwise in a fume hood, and wait until the titanium tetrachloride solution is completely After dispersion in ethanol, a pale yellow solution was obtained at this point.
[0043]Then add 3mmol titanium tetrafluoride to the beaker, stir well for 15 minutes, transfer the resulting suspension into a 100ml autoclave, and react at 180°C for 12 hours. After cooling to room temperature, use 40ml of the obtained solid powder Wash 3 times with absolute ethanol, then wash 3 times with 40ml distilled water, and then wash 2 times with 40ml absolute ethanol. A khaki solid powder was finally obtained, which was then collected by centrifugation, dried at 60°C for 12 hours, and the dried product was taken out, then placed in a muffle furnace and roasted at 180°C for 1 hour, and then groun...
Embodiment 3
[0046] Accurately add 50ml of ethanol to a 100ml beaker, then weigh 1mmol of urea and dissolve it in ethanol, place the beaker on a magnetic stirrer and stir continuously, add 3mmol of titanium tetrachloride dropwise in a fume hood, and wait until the titanium tetrachloride solution is completely After dispersion in ethanol, a pale yellow solution was obtained at this point.
[0047] Then add 3mmol titanium tetrafluoride to the beaker, stir well for 15 minutes, transfer the resulting suspension into a 100ml autoclave, and react at 180°C for 12 hours. After cooling to room temperature, use 40ml of the obtained solid powder Wash 3 times with absolute ethanol, then wash 3 times with 40ml distilled water, and then wash 2 times with 40ml absolute ethanol. A khaki solid powder was finally obtained, which was then collected by centrifugation, dried at 60°C for 12 hours, and the dried product was taken out, then placed in a muffle furnace and roasted at 300°C for 1 hour, then ground t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com