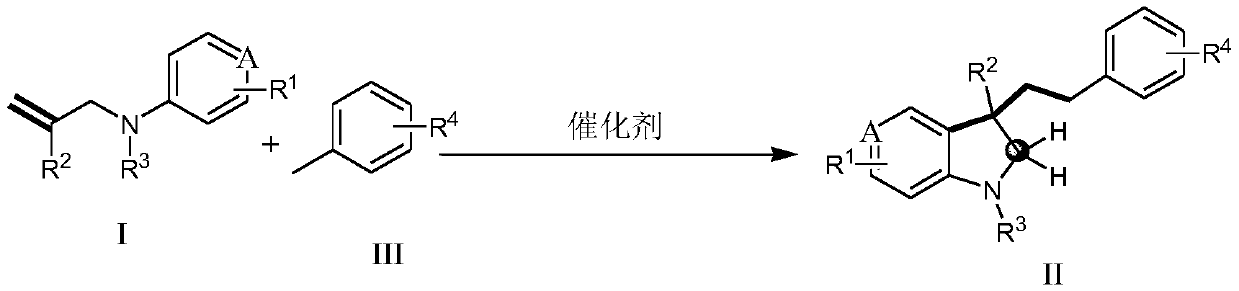
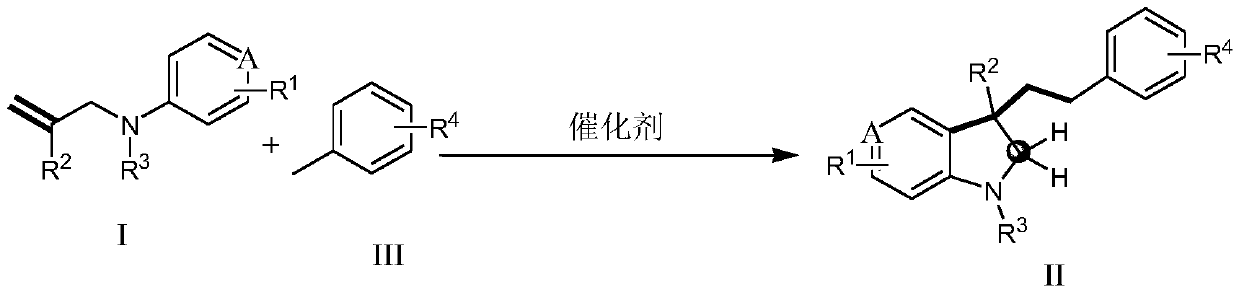
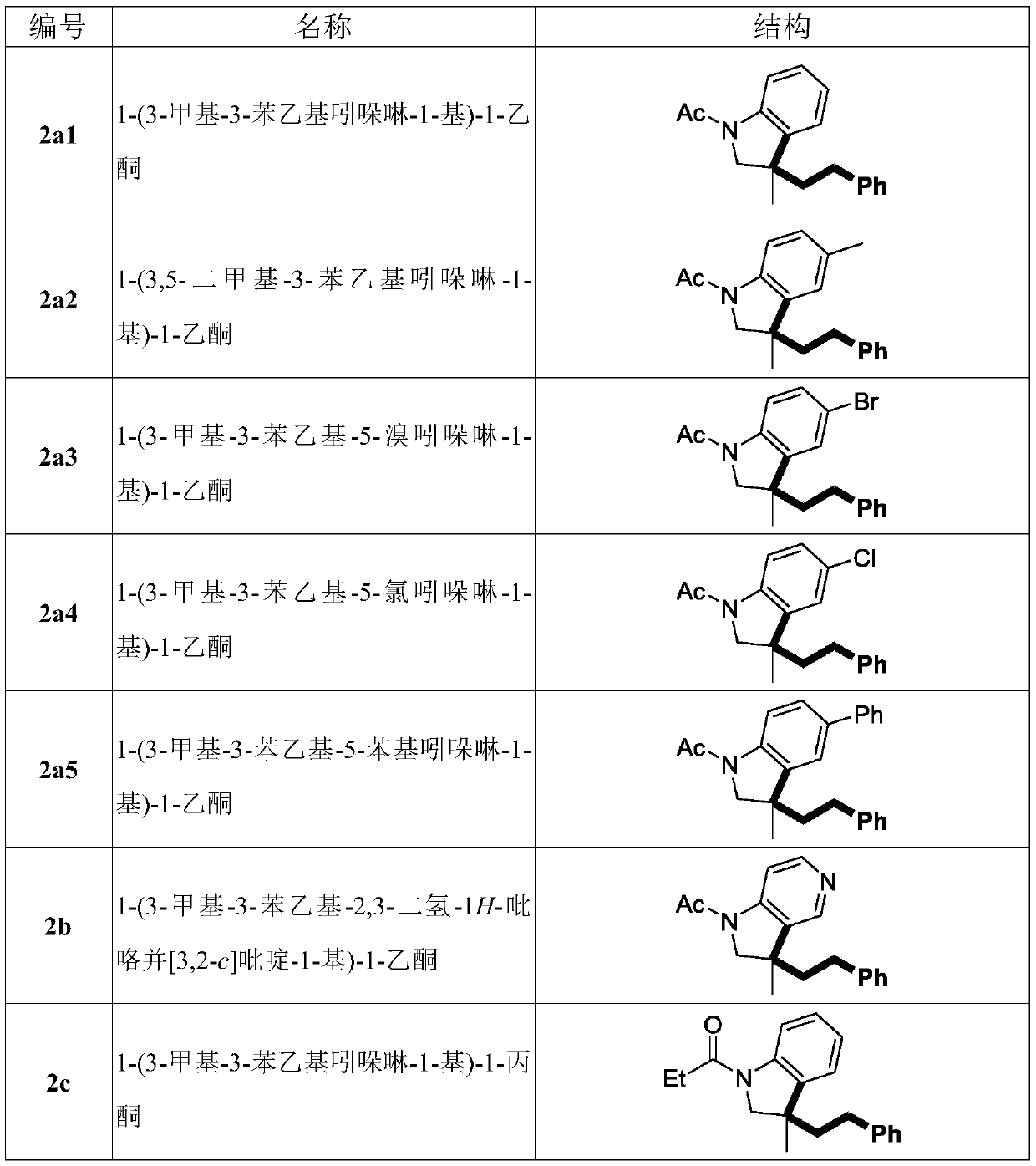
Method for non-activated olefin benzylation synthesis of benzylation indoline compound
A technology of olefin benzyl and phenethyl indoline, applied in organic chemistry and other directions, can solve problems such as functional group intolerance, and achieve the effects of good tolerance, direct synthesis method and wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of non-activated olefins formula I compound (taking 1a2 synthesis as an example), the reaction scheme is as follows:
[0041]
[0042] Operation steps: Add p-toluidine (1.607g, 15.0mmol), CH 2 Cl 2 (50.0 mL), and Et 3 N (4.170 mL, 30.0 mmol) and finally acetyl chloride (1.273 mL, 18.0 mmol) was added. The reaction mixture was stirred at room temperature with a magnetic stirrer. TLC showed that after the raw material p-toluidine was consumed, it was washed with saturated NaHCO 3 solution (100 mL), the reaction was quenched with CH 2 Cl 2 (100.0 mL) was extracted 3 times. The combined organic phases were washed twice with brine (50 mL). The solid obtained by distilling off the organic solvent was washed with a mixture of petroleum ether / ethyl acetate (5:1, volume ratio) to obtain p-methylacetanilide (2.104 g, 94% yield) as a white solid. To a stirred solution of p-methylacetanilide (1.492 g, 10.0 mmol) and NaOH (600 mg, 15.0 mmol) in DMF (30 mL) was a...
Embodiment 2
[0047] Benzylation of non-activated olefins to synthesize benzylated indoline compounds (taking the synthesis of 2a1 as an example), the reaction scheme is as follows:
[0048]
[0049] Operation steps: add N-allyl aniline 1a1 (0.25mmol), MnCl 2 4H 2 O (5 mg, 0.025 mmol), DTBP (73 mg, 0.50 mmol) and solvent toluene (5.0 mL). The mixture was reacted at 140°C for 6h, then washed with saturated Na 2 S 2 o 3 (1.0 mL) and water (10.0 mL) and extracted three times with dichloromethane (10.0 mL). The residue obtained by evaporating the organic solvent was subjected to column chromatography (silica gel as stationary phase, petroleum ether and ethyl acetate as eluent) to obtain the benzylated indoline product 2a1.
[0050] With reference to the above-mentioned Example 2, investigate the influence of the catalyst on the reaction, the details are shown in Table 2 below.
[0051] Table 2 Catalyst's influence investigation on reaction [a]
[0052]
[0053]
[0054] [a] Re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com