Preparation method of coal-bed methane deoxidation catalyst
A technology of deoxidation catalyst and gas bed, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve the problems of high energy consumption, improve reaction efficiency, The effect of solving the loss problem and preventing waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
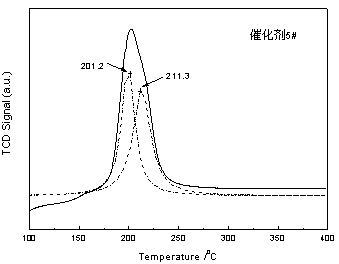
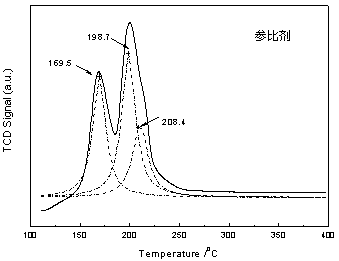
Image
Examples
Embodiment 1
[0032] According to the molar ratio of copper nitrate: aluminum nitrate: methyltriethoxysilane: oxalic acid: 2,5-dihydroxyterephthalic acid: methanol is 0.008: 0.317: 0.0091: 0.034: 0.106: 1, first weigh copper nitrate Add aluminum nitrate and methanol into methanol, stir at 45°C to dissolve it completely, adjust the pH value of the solution to 6; secondly, add methyltriethoxysilane as a silylating agent into the solution; then, mix oxalic acid and 2, 5-dihydroxyterephthalic acid was added into the solution as a compound complexing agent, stirred rapidly to make it evenly mixed, then transferred the solution into a stainless steel reaction kettle lined with polytetrafluoroethylene, and crystallized at 90°C for 8 hours. The crystallized product was washed with ethanol for 5 times, and the obtained material was formed after suction filtration, and then dried at 110° C. for 12 hours. Finally, the obtained material was calcined at 300° C. for 10 h to obtain catalyst 1#.
[0033] ...
Embodiment 2
[0035] According to the molar ratio of manganese nitrate: aluminum nitrate: n-octyltriethoxysilane: succinic acid: 2,5-dihydroxyterephthalic acid: ethanol is 0.029: 0.229: 0.0050: 0.032: 0.076: 1, first weigh Add manganese nitrate and aluminum nitrate into ethanol, stir at 45°C to dissolve them completely, and then adjust the pH of the solution to 6; secondly, add n-octyltriethoxysilane to the solution as a silylating agent; then, add amber Acid and 2,5-dihydroxyterephthalic acid were added to the solution as complex complexing agents, stirred rapidly to make it evenly mixed, then transferred the solution into a stainless steel reaction kettle lined with polytetrafluoroethylene, and kept at 100°C Crystallized for 12h. The crystallized product was washed 5 times with acetone, and the obtained material was formed after suction filtration, and then dried at 110° C. for 12 hours. Finally, the obtained material was calcined at 300° C. for 8 hours to obtain catalyst 2#.
[0036] C...
Embodiment 3
[0038] According to the molar ratio of ferric nitrate: aluminum nitrate: dichlorodimethylsilane: tartaric acid: 2,5-dihydroxyterephthalic acid: isopropanol is 0.028: 0.195: 0.0074: 0.032: 0.065: 1, first weigh nitric acid Iron and aluminum nitrate were added to isopropanol, stirred at 45°C to dissolve them completely, and the pH of the solution was adjusted to 6; secondly, dichlorodimethylsilane was added to the solution as a silylating agent; then, tartaric acid and Add 2,5-dihydroxyterephthalic acid into the solution as a compound complexing agent, stir rapidly to make it evenly mixed, then transfer the solution into a stainless steel reaction kettle lined with polytetrafluoroethylene, and crystallize at 110°C 16h. The crystallized product was washed with ethanol for 5 times, and the obtained material was formed after suction filtration, and then dried at 110° C. for 12 hours. Finally, the obtained material was calcined at 300°C for 16 hours to obtain catalyst 3#.
[0039]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com