Method for efficient selective preparation of fatty diacid mono-tert-butyl ester
A technology of fatty diacid and mono-tert-butyl ester, which is applied in the field of biomedicine, can solve the problems of selectivity, cost, and unsatisfactory yield, and achieve the effects of wide applicability, good selectivity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0068] Preparation Example 1: Synthesis of mono-tert-butyl octadecanedioate
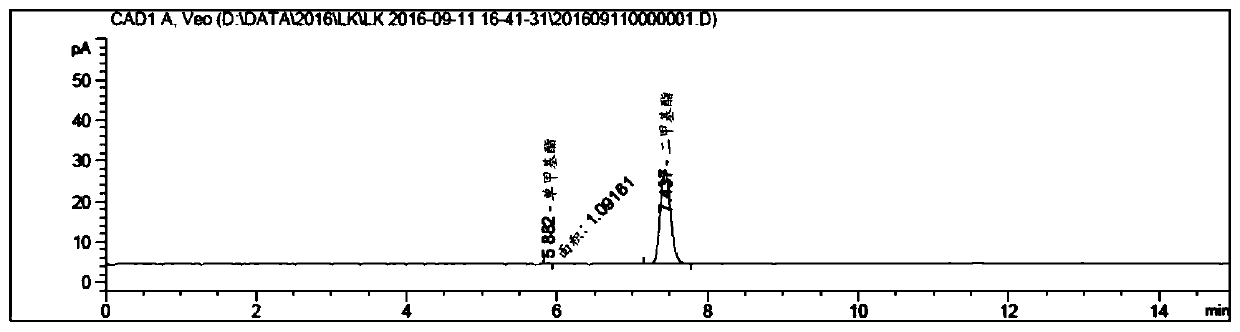
[0069] Step 1: Under nitrogen protection, 1.30 kg of octadecanedioic acid, 130 g of concentrated sulfuric acid and 7.2 L of methanol were heated to reflux for 8 hours, and the reaction progress was detected by HPLC. After the reaction was completed, the reaction temperature was cooled to 0° C. to 5° C., a large amount of solids precipitated, stirred for 2 hours, filtered, and the wet cake was dried to obtain 1.41 kg of dimethyl octadecanedioate, with a yield of 100%. The obtained product was identified by HPLC.
[0070] see figure 1 , the integration results measured by HPLC are shown in Table 1 below.
[0071] Table 1
[0072]
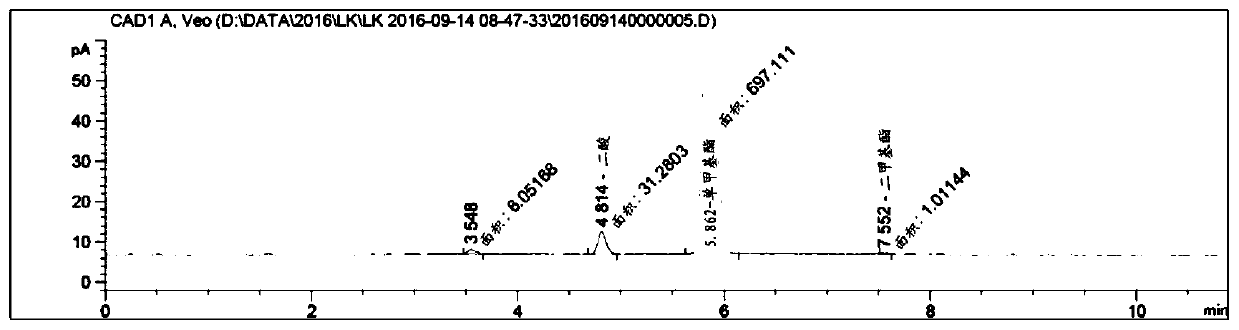
[0073] Step 2: Mix 1.41kg of dimethyl octadecanedioate solid obtained in step 1 with 704g of barium hydroxide octahydrate and 15L of methanol, and then stir the mixed suspension at 20°C to 25°C for 24 hours, The reaction solution was detected by HPLC. After the react...
preparation example 2
[0086] Preparation Example 2: Synthesis of Eicosanedioic Acid Mono-tert-Butyl Ester
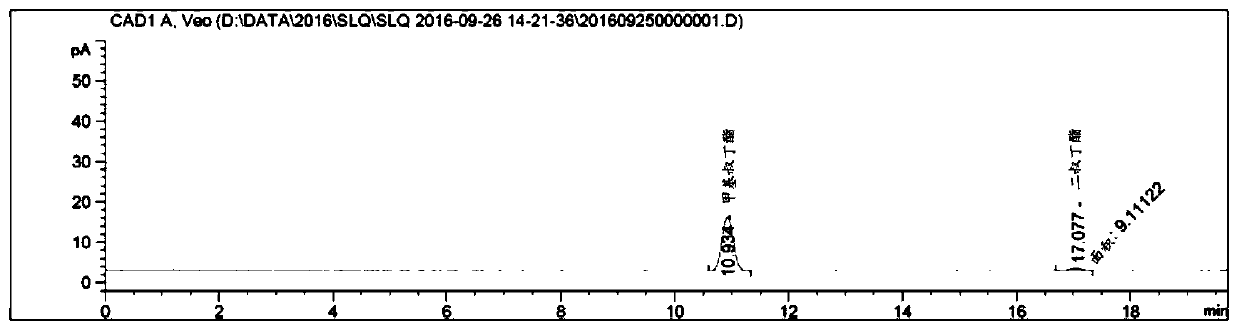
[0087] Step 1: Under nitrogen protection, 3.0 kg of eicosanedioic acid, 282 g of concentrated sulfuric acid and 20 L of methanol were heated to reflux for 8 hours, and the reaction progress was detected by HPLC. After the reaction was completed, the reaction temperature was cooled to 0°C to 5°C, a large amount of solids were precipitated, stirred for 2 hours, filtered, and the wet cake was dried to obtain 3.20 kg of dimethyl eicosanedioate with a yield of 98%. The obtained product was identified by HPLC.
[0088] table 5
[0089]
[0090] Step 2: Add 3.20 kg of dimethyl eicosanedioate solid obtained in step 1 to 1.6 kg of barium hydroxide octahydrate and 32 L of methanol, and let the suspension stir at 20°C to 25°C for 24 hours, and detect the reaction by HPLC liquid. After the reaction was completed, the barium salt of the product was filtered out. Mix the barium salt with 30L of wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com