Organic electroluminescent material and application thereof
An organic, selected technology, applied in the fields of light-emitting materials, organic chemistry, circuits, etc., can solve the problems of lack of host materials, high driving voltage, unfavorable carrier injection and transport balance, etc., to achieve high mobility and reduce work. Effects of voltage and high carrier transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
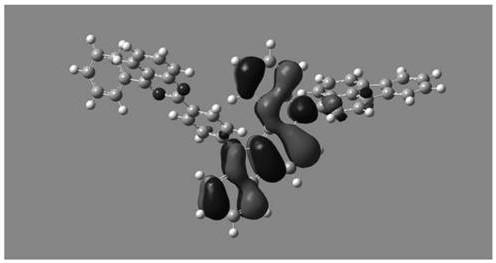
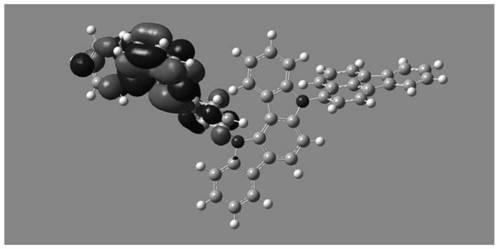
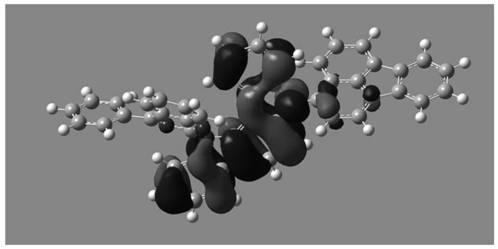
Image
Examples
Synthetic example 1
[0060] Synthesis Example 1 is a preparation example of an intermediate of the present invention, and the intermediate is a structure shown in formula M1:
[0061]
[0062] Utilize the M1 intermediate, select appropriate synthetic raw materials as required, and carry out the reaction by known methods to obtain compounds of the present invention with different structures. Other compounds can be obtained by suzuki coupling reaction between M1 and different raw materials.
[0063] Synthesis Example 1: Synthesis of Intermediate M1
[0064]
[0065] In a 250mL three-neck flask, add compound A (1.0g, 3.9mmol), compound B (1.2g, 4.8mmol), Pd 2 (dba) 3 (0.04g, 0.039mmol), sodium tert-butoxide (0.75g.7.8mmol), tri-tert-butylphosphine tetrafluoroborate (0.02g, 0.078mmol) and 30ml of xylene, pump and fill nitrogen 3 times, in nitrogen Under protection, the temperature was raised to reflux temperature. After reacting overnight, the temperature was lowered to room temperature, dilu...
Synthetic example 2
[0066] Synthesis example 2: Synthesis of the shown compound P1
[0067]
[0068] Add compound M1 (1.0g, 2.2mmol), compound C (0.90g, 2.64mmol), potassium carbonate (0.91g, 6.6mmol) and 30ml DMF into a 250mL three-necked flask, pump and charge nitrogen 3 times, and raise the temperature under nitrogen protection After reacting overnight at 130°C, cool down to room temperature, pour the reaction solution into water to precipitate precipitates, filter the precipitates, dissolve the filter cake in toluene, pass through a silica gel column, and then recrystallize with toluene to obtain 1.3 g of a bright yellow solid. rate 80%. MS (m / e): 763, 1 H NMR (300MHz, CDCl 3 ): 9.30(2H), 9.15(2H), 8.56(2H), 8.53(2H), 8.43(2H), 8.16(1H), 8.11(2H), 8.02(2H), 7.95(2H), 7.80(3H ), 7.70(6H), 7.34(2H), 7.26(3H), 7.16(2H), elemental analysis: C(86.5%), N(9.2%), H(4.3%).
Synthetic example 3
[0069] Synthesis example 3: Synthesis of the shown compound P19
[0070]
[0071] The preparation method was the same as that of P1, except that compound C was replaced by compound D to obtain 1.4 g of a bright yellow solid product with a yield of 81%. MS (m / s): 762, 1 H NMR (300MHz, CDCl 3 ): 8.56(2H), 8.43(2H), 8.24(1H), 8.13(3H), 7.95(4H), 7.82(7H), 7.68(4H), 7.52(4H), 7.42(2H), 7.34(2H ), 7.26(2H), 7.24(1H), elemental analysis: C (88.2%), N (7.3%), H (4.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com