Glutamine boron trifluoride analogue with elongated carbon chain
A technology of amide derivatives and compounds, which is applied in the field of nuclear medicine diagnosis and treatment, can solve the problems of insufficient stability of glutamic acid probes, and achieve the effects of improving pharmacokinetic characteristics, prolonging the half-life of drug activity, and excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
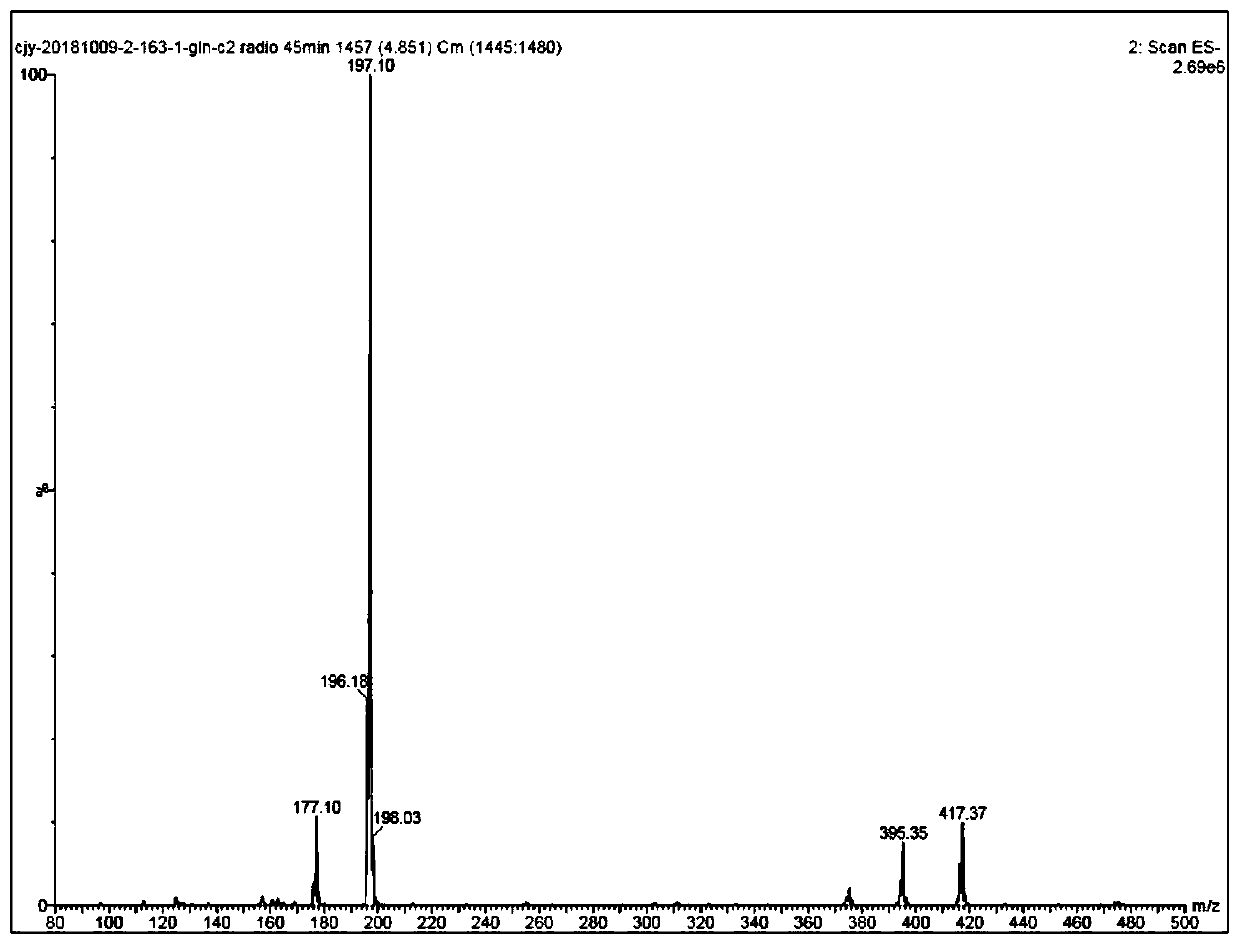
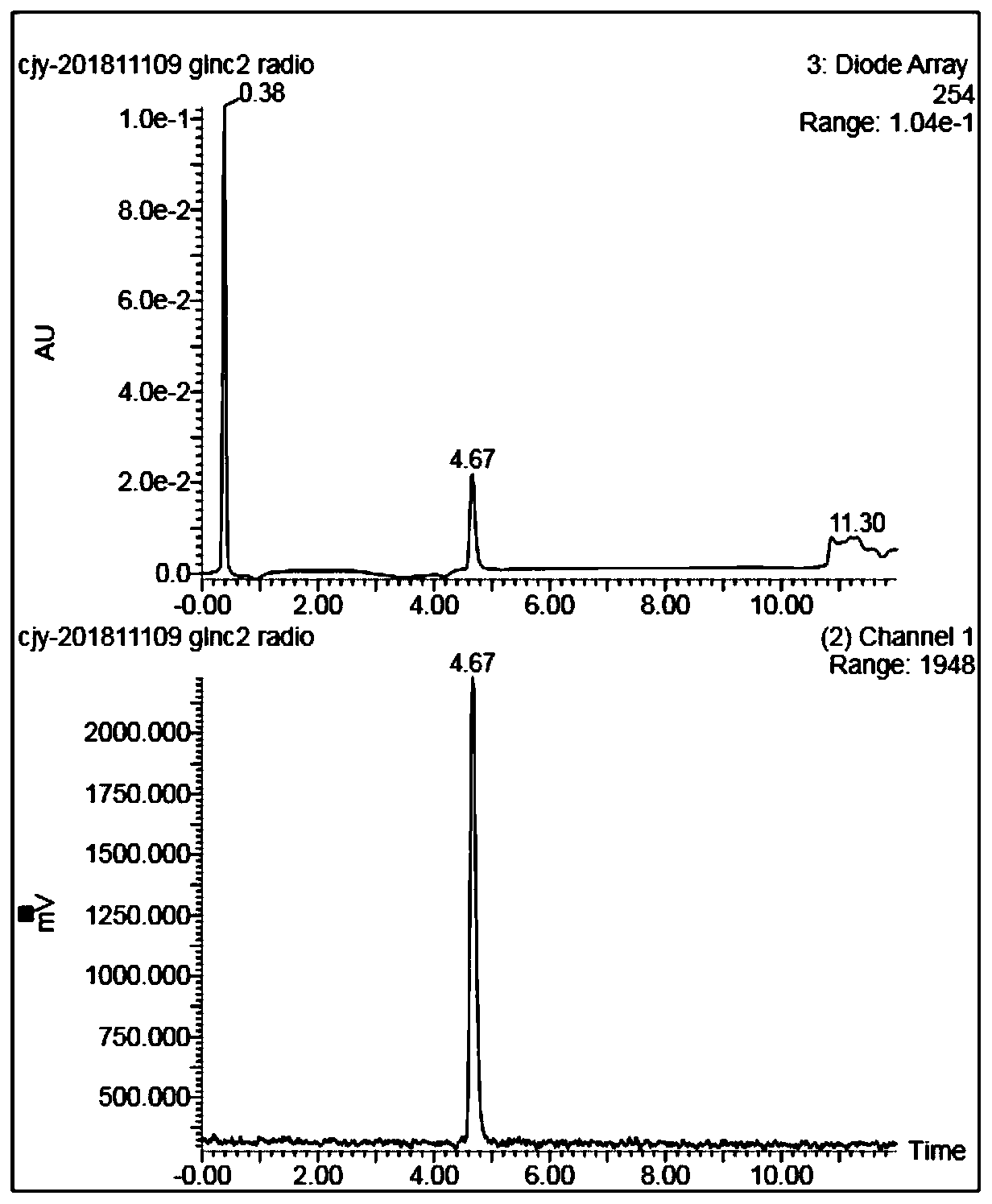
[0054] This embodiment provides a carbon chain-extended glutamine boron trifluoride molecular probe, and the reaction process for its preparation is as follows:
[0055]
[0056] Concrete synthetic steps are:
[0057] The 1st step, the synthesis of compound 1:
[0058] Dissolve monomethyl adipate (1eq) with tritylamine (1qe), O-benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroboric acid (TBTU, 1eq) In dichloromethane, react overnight at room temperature. The reaction was quenched by adding water, extracted with ethyl acetate, dried and the solvent was removed under reduced pressure. Column chromatography separated to obtain the corresponding tribenzamide compound, namely compound 1.
[0059] NMR results of compound 1: 1H NMR (400MHz, CDCl3, ppm): δ7.35–7.08(m,15H),6.58(s,1H),3.65(s,3H),2.45–2.23(m,4H), 1.75–1.44 (m, 4H).
[0060] The 2nd step, the synthesis of compound 2:
[0061] Dissolve compound 1 (1eq) in tetrahydrofuran, add lithium borohydride (2qe), and re...
experiment example 1
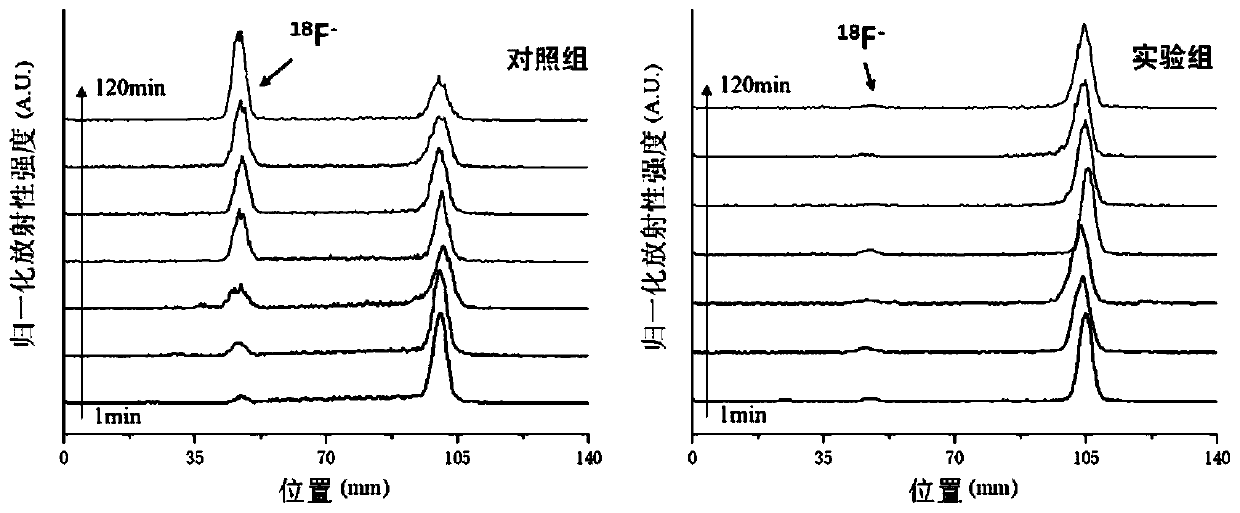
[0079] Experimental Example 1 Stability Detection
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com