Refining method of sodium taurocholate
A technology of sodium taurocholate and a purification method, applied in the field of medicinal chemistry, can solve problems such as low purity and low yield, and achieve the effects of good product purity, high yield and reducing solvent volatilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of sodium taurocholate: Measure 5L of N,N'-dimethylformamide into a reaction kettle, raise the temperature to 90°C, add cholic acid, and stir until all the solids are dissolved. Add 0.7kg 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline, 0.3kg triethylamine, 0.36kg taurine, and stir for 2 hours at 90°C. After cooling down to room temperature, 25 L of methyl tert-butyl ether was added to the reaction kettle to precipitate a solid, which was filtered. The resulting solid was dissolved in 5L of methanol, 0.07kg of sodium hydroxide was added, and stirred at room temperature for 1 h. Add 25L of methyl tert-butyl ether into the reaction kettle, filter, and dry under vacuum at 60°C to obtain crude sodium taurocholate.
Embodiment 2
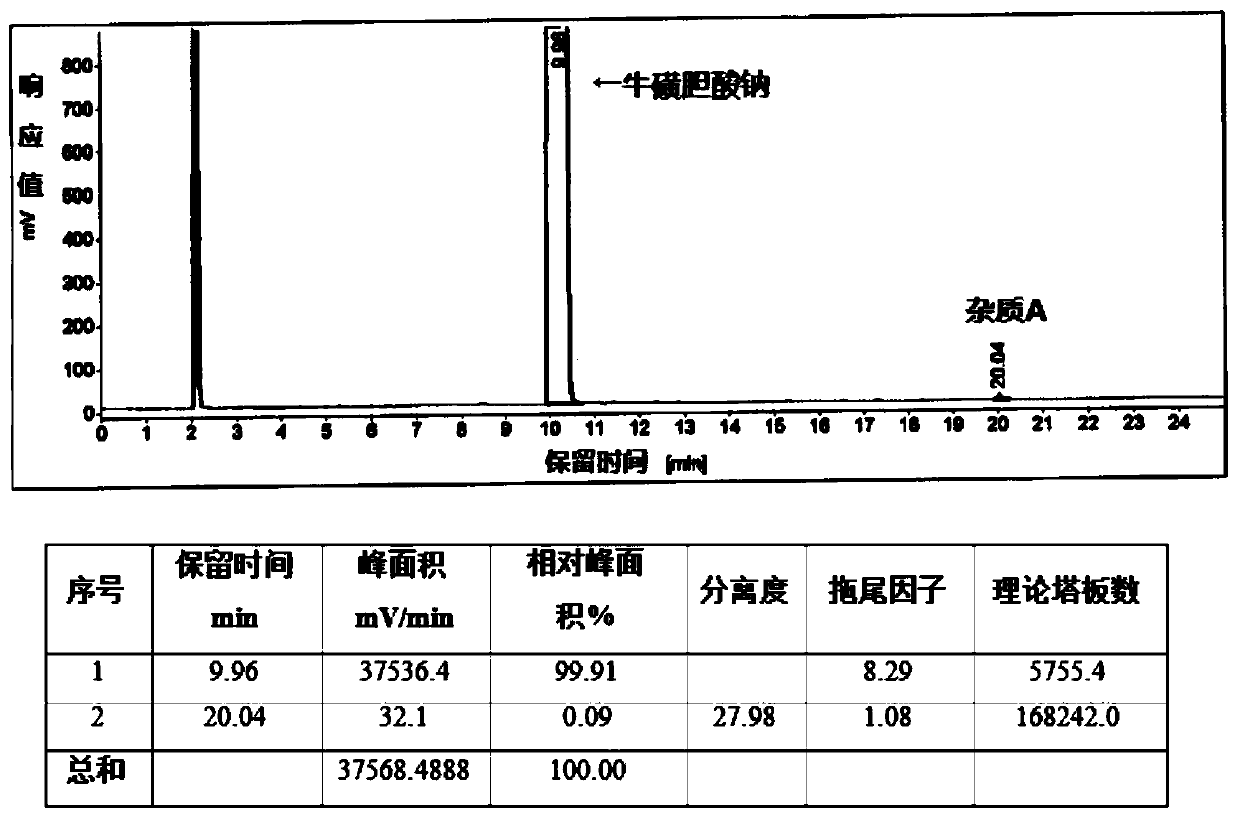
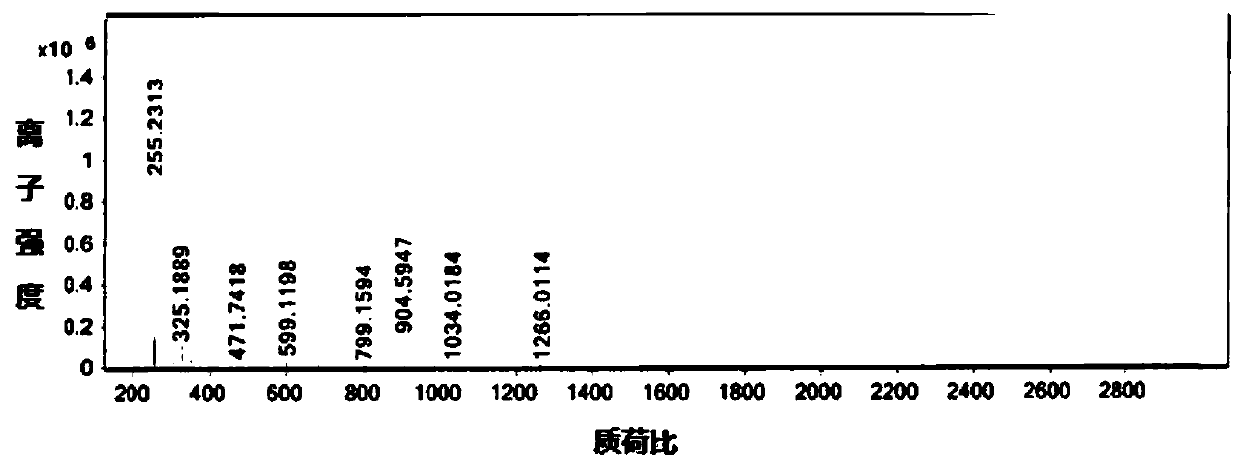
[0050] Add 20g of crude sodium taurocholate (self-made, HPLC content 93.78%, maximum simplex 2.53%) and 80ml of methanol into a 250ml reaction bottle, heat to 50°C to dissolve, add 120ml of ethyl acetate while it is hot, cool to 25°C and stir Crystallized, filtered, and dried under reduced pressure to obtain 18.2 g of sodium taurocholate finished product, with a yield of 91%, an HPLC content of 99.91%, and a maximum simple A content of 0.09%. as attached figure 1 As shown: the peak with a peak time of 9.96 min is sodium taurocholate, and the peak with a peak time of 20.04 min is impurity A, and the mass spectrum is as follows figure 2 As shown, the molecular weight is 904.59.
Embodiment 3
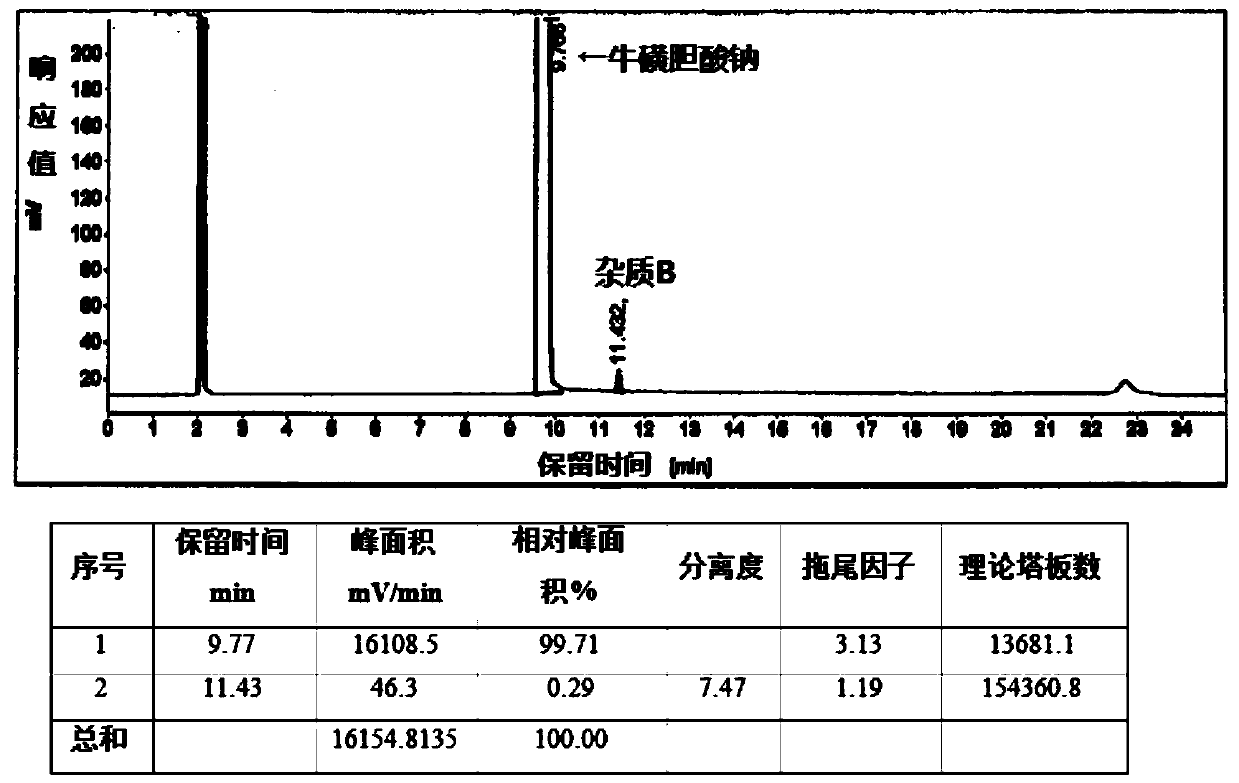
[0052] Add 20g of crude sodium taurocholate (self-made, HPLC purity 93.78%, maximum 2.53%) and 60ml of methanol into a 250ml reaction bottle, heat to 50°C to dissolve, add 120ml of ethyl acetate while it is hot, cool to 25°C and stir Crystallized, filtered, and dried under reduced pressure to obtain 19 g of sodium taurocholate finished product, with a yield of 95%, an HPLC content of 99.71%, and a maximum simple impurity B content of 0.29%. as attached image 3 Shown: the peak with a peak time of 9.77 min is sodium taurocholate, and the peak with a peak time of 11.43 min is impurity B: sodium taurochenodeoxycholate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com