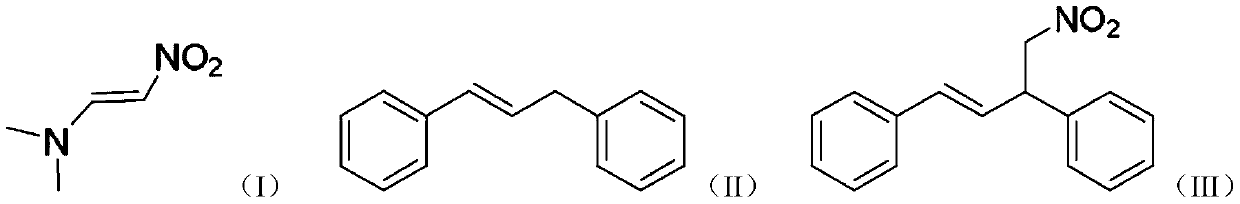
Method for preparing (E)-1, 3-diphenyl-4-nitro-1-butene
A technology of diphenylpropylene and diphenyl, which is applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as long reaction time and high reaction temperature, and achieve the effects of simple operation, high yield and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] At room temperature, dissolve 0.1943g (1.0mmol) (E)-1,3-diphenylpropene in 3mL 1,2-dichloroethane, add 0.1362g (0.6mmol) DDQ, stir for 10 minutes, add 0.05806g (0.5mmol) (E)-1-dimethylamino-2-nitroethylene was reacted for 2 hours, and then 0.1ml of 1mol / L hydrochloric acid aqueous solution was added to the reaction liquid, and the reaction was continued for 0.5 hours. After the reaction is over, add 30 g of 200-300 mesh silica gel to the reaction solution, remove the solvent under reduced pressure, and pass through the column. The developer used is petroleum ether: ethyl acetate = 30:1, and the colorless oily (E)-1,3 -Diphenyl-4-nitro-1-butene, yield 78%.
Embodiment 2
[0021] At room temperature, dissolve 0.1943g (1.0mmol) (E)-1,3-diphenylpropene in 3mL 1,2-dichloroethane, add 0.1362g (0.6mmol) DDQ, stir for 10 minutes, add 0.05806g (0.5mmol) (E)-1-dimethylamino-2-nitroethylene was reacted for 2 hours, and then 0.1ml of 1mol / L formic acid aqueous solution was added to the reaction liquid, and the reaction was continued for 0.5 hours. After the reaction was completed, 30 g of 200-300 mesh silica gel was added to the reaction solution, the solvent was removed under reduced pressure, and the solvent was passed through the column. The developer used was petroleum ether: ethyl acetate = 30:1, and the colorless oily (E)-1 was obtained by separation. 3-Diphenyl-4-nitro-1-butene, yield 75%.
Embodiment 3
[0023] At room temperature, dissolve 0.1943g (1.0mmol) (E)-1,3-diphenylpropene in 2mL 1,2-dichloroethane, add 0.1362g (0.6mmol) DDQ, stir for 10 minutes, add 0.05806g (0.5mmol) (E)-1-dimethylamino-2-nitroethylene was reacted for 2 hours, and then 0.1ml of 1mol / L acetic acid aqueous solution was added to the reaction liquid, and the reaction was continued for 0.5 hours. After the reaction was completed, 30 g of 200-300 mesh silica gel was added to the reaction liquid, the solvent was removed under reduced pressure, and the solvent was passed through the column. The developer used was petroleum ether: ethyl acetate = 30:1, and (E)-1 was separated as a colorless oil. 3-Diphenyl-4-nitro-1-butene, yield 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com