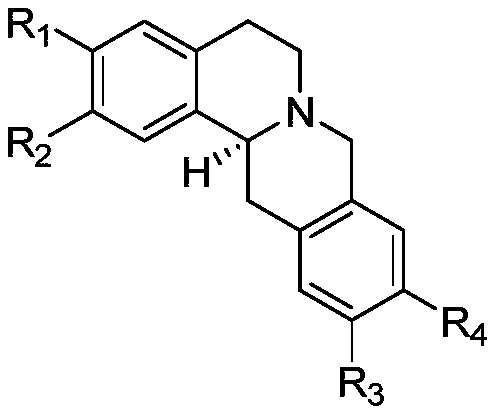
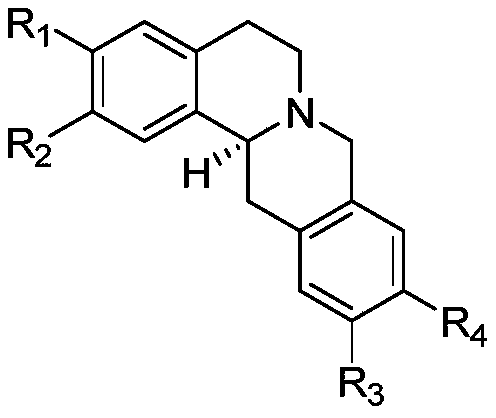
Application of protoberberine-type compound with TDO selective inhibition activity in medicament preparation
A protoberberine and compound technology, which is applied in the field of protoberberine compounds and can solve problems such as no protoberberine compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
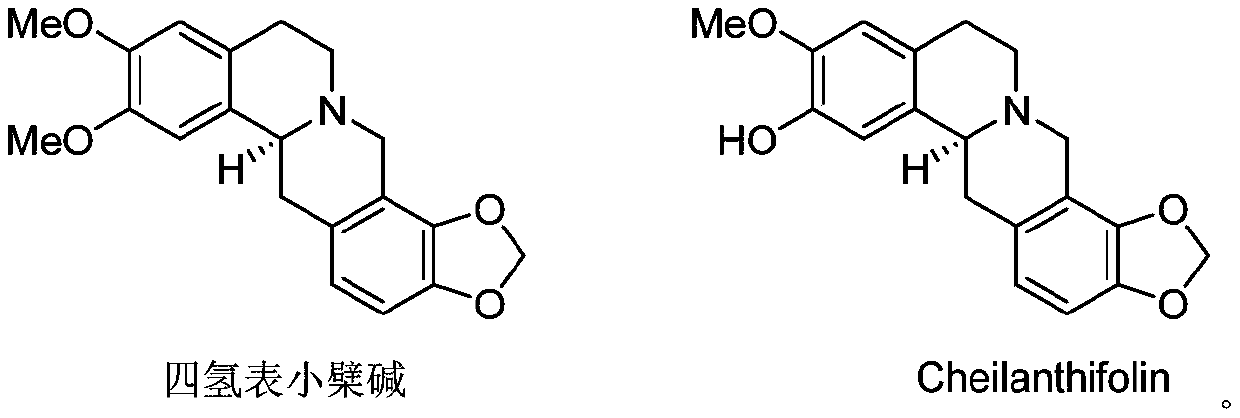
[0021] Preparation of Compounds Tetrahydroepiberberine and Coryrydine Cheilanthifolin:
[0022]
[0023] Dried ivy (Sinomeniumacutum (Thunb.) Rehd.et Wils.) 10kg, crushed and extracted with methanol 3 times (30L×3) at room temperature, soaked for 24h each time. The extracts were combined, the organic solvent was recovered by distillation under reduced pressure, the pH value was adjusted to 1-2 with 3% hydrochloric acid, and extracted three times with ethyl acetate. The aqueous phase was adjusted to pH 9-10 with aqueous ammonia, extracted three times with chloroform, and concentrated to obtain 144 g of total alkali. The total alkali was eluted by basic alumina (100-200 mesh, 2kg), petroleum ether-acetone (7:1-1:1), chloroform-methanol (100:1-1:1) gradient elution, after detection Three fractions (Fr1-Fr3) were combined. Fraction 3 (26 g) was eluted with a gradient of 10-100% methanol / water through a medium-pressure MCI column, and 7 sub-fractions (Fr2.1-Fr2.7) with increas...
Embodiment 2
[0027] experimental method:
[0028] 1.1 Protein sample preparation for TDO
[0029] References for gene cloning, protein expression and purification of human TDO (Meng, Wu et al.2014). References for gene cloning, protein expression and purification of human IDO (Nelp, Kates et al. 2018). Based on literature reports, slightly adjusted according to experimental conditions.
[0030] Protein Concentration Determination
[0031] Using the bovine serum protein standard curve as a reference, the protein concentration was detected by BCA Protein Assay Kit. In each detection process, different dilution concentrations are used for detection at the same time to ensure the error in the concentration detection process.
[0032] 1.2 Activity experiment
[0033] The reaction system is 200uL, including 20uL of 0.5M potassium phosphate buffer (pH 6.5), 40uL of 0.2M ascorbic acid, 8uL of 0.5mM methylene blue, 8uL of 5mg / ml catalase, and 15uL of L-tryptophan , 120uL ddH 2 O. The final ...
Embodiment 3
[0049] Tablet: Mix 10 mg of the compound obtained in Example 1, add 180 mg of lactose, 55 mg of starch and other auxiliary materials and mix evenly, moisten it with water, make granules and dry, then add 5 mg of magnesium stearate and mix evenly, and then press into tablets. Tablets weigh approximately 250mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com