Dearomatization-based novel 1,4 addition type Ugi reaction
A dearomatization and reaction technology, applied in the field of Ugi reaction, can solve problems such as poor substrate applicability, and achieve the effect of good substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
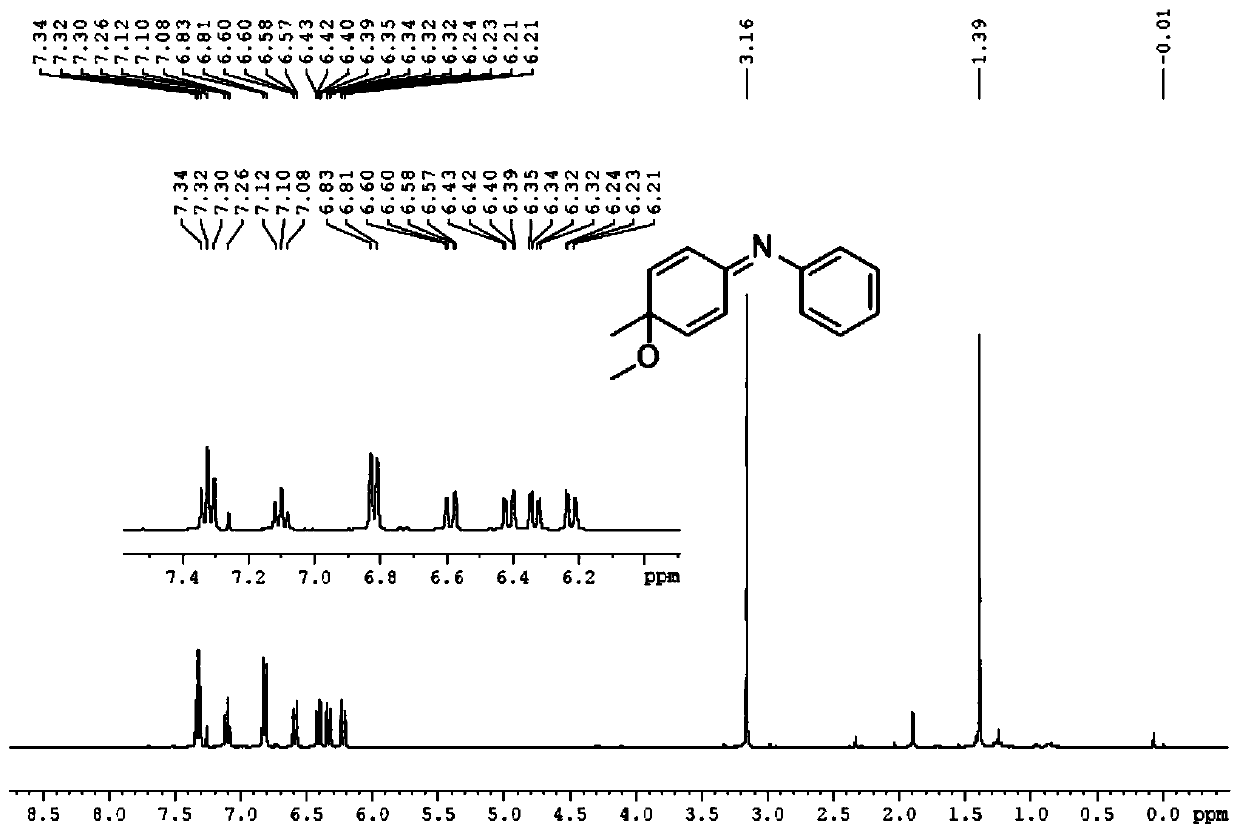
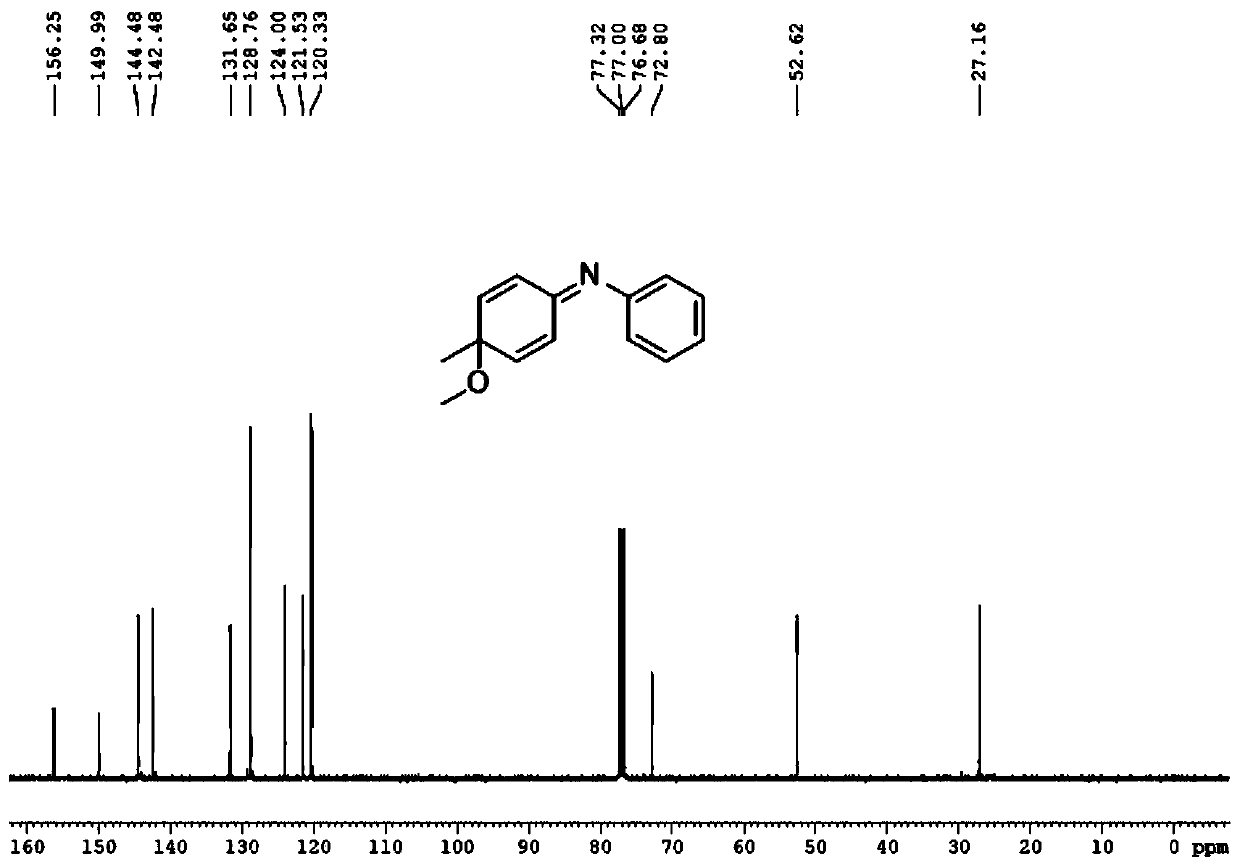
[0046]
[0047] First take a 50mL round bottom flask, add 10.0mL methanol at room temperature, add the pure compound 4-methyl-N-phenylaniline (1.0mmol) under stirring, cool to -40°C, add 320mg (1.0mmol) iodobenzene diacetic acid, stirred for 30 min, transferred the reaction solution to room temperature, stirred for 10 min, and monitored the progress of the reaction with TLC until the reactant disappeared, and the reaction was completed.
[0048] After the reaction finishes, transfer the reaction solution to a 50ml separatory funnel with ethyl acetate, extract three times with ethyl acetate, combine the organic phases, wash three times with saturated aqueous sodium chloride solution, dry the organic phase with anhydrous sodium sulfate to remove water, The filtered filtrate was rotary evaporated to dryness under reduced pressure to obtain a crude product. The crude product was purified by silica gel column chromatography, and the eluent was V 石油醚 :V 乙酸乙酯 =30:1, the product ...
Embodiment 2
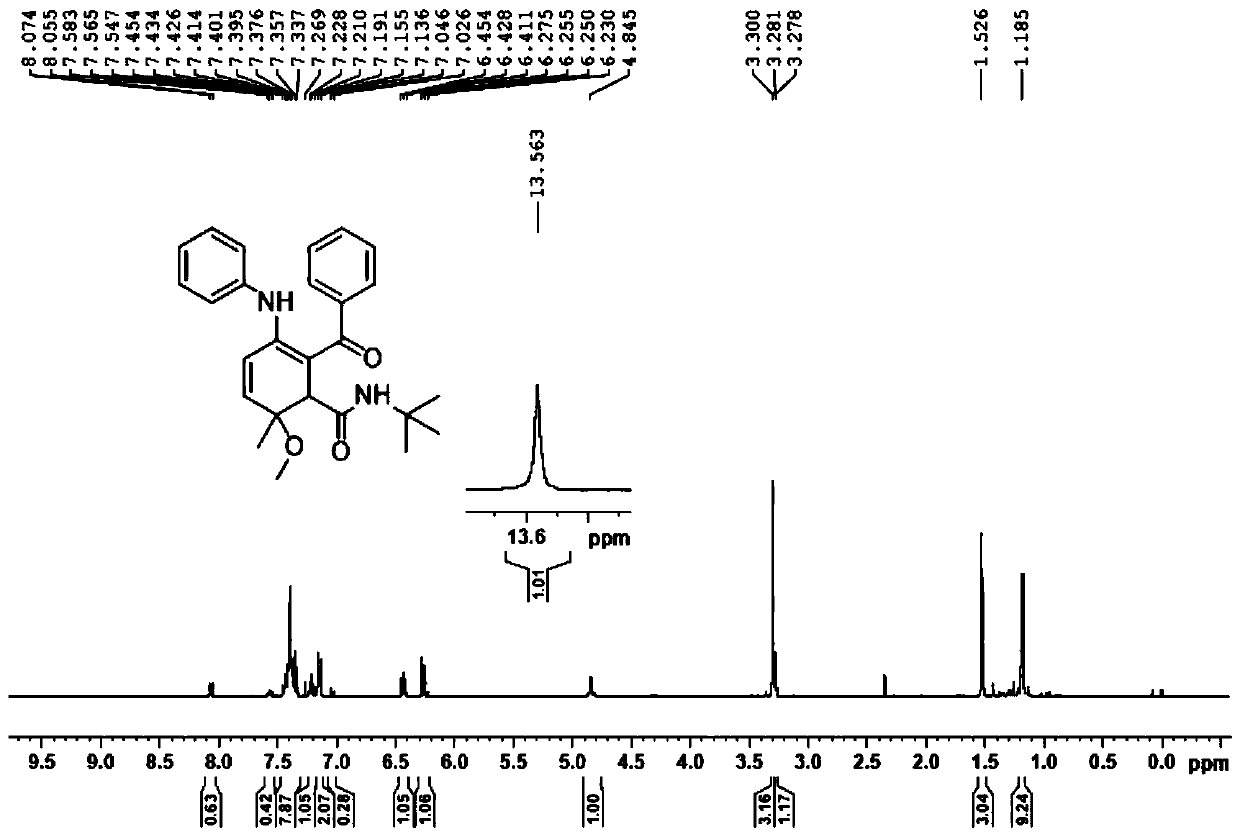
[0053]
[0054] First, take a 15ml pressure-resistant reaction tube, add 2 mL of trifluoroethanol, then add imine 2a (1.0 mmol), benzoic acid (2.0 mmol), and tert-butylisonitrile (2.0 mmol) into the pressure-resistant reaction tube in sequence, and tighten The stopper was stirred for 10 minutes, and the reaction bottle was moved into a 60° C. oil bath, and the progress of the reaction was monitored with a TLC (thin layer chromatography) 5×2 cm silica gel plate until the end of the reaction.
[0055] After the reaction was completed, the reaction solution was transferred to a 50 mL round-bottomed flask with methanol solvent, and evaporated to dryness under reduced pressure to obtain a crude product. The crude product was purified by silica gel column chromatography, and the eluent was V 石油醚 :V 乙酸乙酯 =20:1-5:1, the pure product 5a was obtained, and the yield was calculated.
[0056] 1 H NMR (400MHz, CDCl 3 )δ13.56(s,1H),7.45-7.33(m,7H),7.22(d,J=8.0Hz,1H),7.15(d,J=8.0Hz,2H)...
Embodiment 3
[0060]
[0061] Take a 50ml reaction eggplant-shaped bottle, carry out anhydrous and oxygen-free operation, at room temperature, under the protection of argon, add 192mg (2mmol, 2equiv) of sodium tert-butoxide, 91.5mg (0.05mmol) of tris(dibenzylideneacetone) dipalladium , 0.05equiv), 4ml of anhydrous toluene was added under stirring, and then 93.8ul of tri-tert-butylphosphine (0.2mmol, 0.2equiv) was added and stirred for 2 minutes. Add 4-bromoethylbenzene (1.2 mmol, 1.2 equiv) and aniline 1.0 mmol, 1 equiv) for the last time, and then transfer the reaction system to a 50° C. oil bath for 1 h. TLC monitoring until the end of the reaction. Slowly add water to quench, add ethyl acetate (10mL×3) for extraction, combine the organic phases, wash with saturated sodium chloride (30mL×3), dry over anhydrous sodium sulfate and spin dry to obtain a crude product. The crude product was purified by silica gel column chromatography, and the eluent was V 石油醚 :V 乙酸乙酯 =50:1, the pure com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com