Method for preparing methyl furoate by directly oxidizing and esterifying furfural
A technology of oxidative esterification and methyl furoate, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve the problem of ineffective utilization and catalyst deactivation , active point coverage and other issues, to achieve significant industrial application value, simplify the reaction process, and easy to recycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
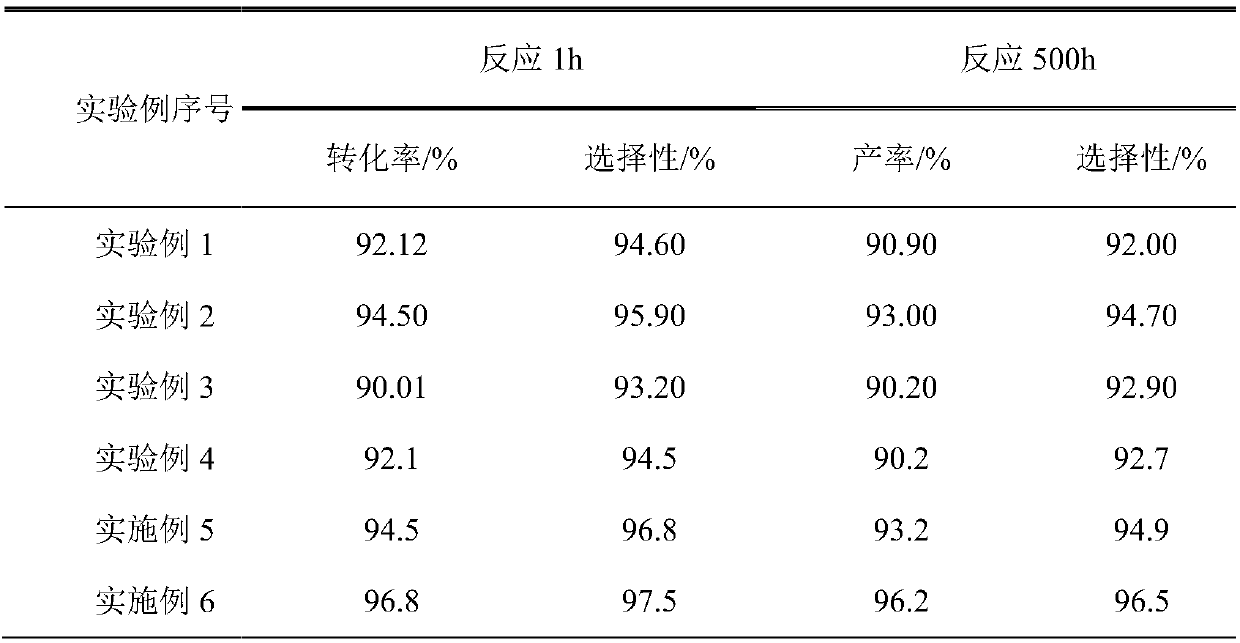
Examples
preparation example Construction
[0023] Preparation of cobalt-based composite particle load carrier:
[0024] Mix 10g of 30% silica sol (pH=4.5), 1.96g of magnesium hydroxide, 7.5g of zirconium nitrate, 3g of concentrated nitric acid with a concentration of 80%, and 60mL of deionized water at 25°C. A homogeneous solid solution suspension was obtained, water was removed by rotary evaporation, and vacuum-dried at 80° C. to obtain a white powdery solid. Put the solid in a tube furnace, heat up and roast under nitrogen, heat up at 30-300°C for 3h (heating rate 1.5°C / min), keep at 300°C for 3h, heat up at 300-600°C for 3h (heating rate 1.7°C / min) , keep at 600°C for 3h. After natural cooling, a SiO2-MgO-ZrO2 metal composite oxide carrier is obtained.
Embodiment 1
[0026] Preparation of cobalt-based composite particle loading material A:
[0027] Add 1 g of SiO to the reactor sequentially 2 -MgO-ZrO 2 Carrier, 0.3g urea, 21.0mg chloroauric acid, 0.18g cobalt nitrate, and 70mL deionized water were mixed evenly, stirred and reacted at 80°C for 4h, and the mixture was cooled to room temperature, filtered under reduced pressure to obtain a solid, and the solid was vacuum-dried at 80°C 1h, the solid was calcined in a muffle furnace at 450°C for 3h. The cobalt-based composite particle load A was obtained after natural cooling.
[0028] The specific method for preparing methyl furoate by oxidative esterification of furfural is: 100mg of cobalt-based composite particle load A (0.1mol%), and the methanol solution (containing methanol 7.5mL) dissolved with 0.4805g (5mmol) furfural is added successively to the In a 25mL glass-lined autoclave, after three times of oxygen replacement, pressurize to 1.0MPa, stir at 500rpm, react at 130°C for 4h, co...
Embodiment 2
[0030] Preparation of cobalt-based composite particle load B:
[0031] Add 1 g of SiO to the reactor sequentially 2 -MgO-ZrO 2Carrier, 0.3g urea, 21.0mg chloroauric acid, 0.36g cobalt nitrate, and 70mL deionized water were mixed evenly, stirred and reacted at 80°C for 4h, and the mixture was cooled to room temperature, filtered under reduced pressure to obtain a solid, and the solid was vacuum-dried at 80°C 1h, the solid was calcined in a muffle furnace at 450°C for 3h. The cobalt-based composite particle load B was obtained after natural cooling.
[0032] The specific method of furfural oxidative esterification to prepare methyl furoate is: 100mg of cobalt-based composite particle load B (0.1mol%), dissolved in methanol solution (containing methanol 7.5mL) of 0.4805g (5mmol) furfural is added successively to the In a 25mL glass-lined autoclave, after three times of oxygen replacement, pressurize to 1.0MPa, stir at 500rpm, react at 130°C for 4h, cool to room temperature, de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com