Polycrystal of pteridinone compounds or their salts and preparation method and application thereof
A compound and crystal form technology, applied in the field of medicine, can solve problems such as enlargement, and achieve the effects of good water solubility, high stability, and good crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 4-amino-2-butoxy-8-((6-(4-methylpiperazin-1-yl)pyridin-3-yl)methyl)-7,8-dihydropteridine- Preparation of 6(5H)-ketone
[0077]
[0078] The first step: tert-butyl 4-(5-formylpyridin-2-yl)piperazine-1-carboxylate
[0079] The compound 6-bromonicotinaldehyde (1.0g, 5.376mmol), N-Boc-piperazine (1.0g, 5.376mmol) and DIEA (2.5mL) were added to 10mL of DME, heated to 120°C and stirred for 6.5 hours. Water was added, extracted with EA, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 1.4 g of a yellow solid (yield 89.7%).
[0080] The second step: tert-butyl 4-(5-((ethoxycarbonylmethyl-amino)methyl)pyridin-2-yl)piperazine-1-carboxylate
[0081] Compound 4-(5-formylpyridin-2-yl)piperazine-1-carboxylic acid tert-butyl ester (1.2g, 4.12mmol), glycine ethyl ester hydrochloride (1.2g, 8.60mmol) and cyanoboron Sodium hydride (0.6g, 9.548mmol) was added into 30mL methanol system, and the reaction was stirred at 20°C for 3 h...
Embodiment 2
[0097] The preparation of embodiment 2 crystal form I
[0098] Method 1: Weigh 0.1 g of the compound sample of formula I and disperse it in 5 ml of methanol, suspend and stir for 24 hours, filter and collect the crystals, dry in vacuum at 50°C for 1 hour to obtain a white powder, which is the crystal form I of the present invention, which 1 HNMR spectrum such as Figure 4 shown.
[0099] Structural characterization of Form I
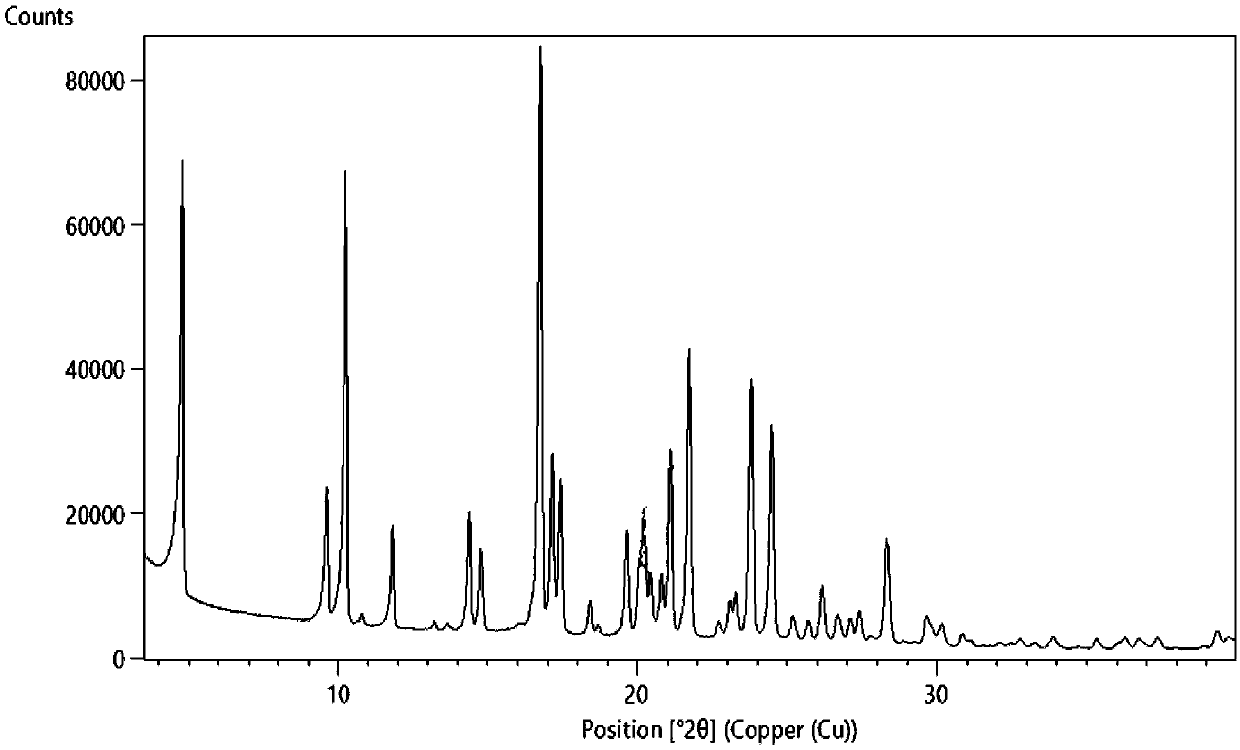
[0100] X-ray powder diffraction (XRPD)
[0101] Adopt X`Pert3 Powder powder diffractometer to obtain the XRPD pattern of crystal form I, such as figure 1 As shown, the instrument is irradiated with Cu palladium and detected using Absolute scan at room temperature. The detection range is from 3.5° to 40°, the step size is 0.013, the dwell time is 50s, and the scan is performed once. The XRPD crystal form characterization data of Form I are shown in Table 1.
[0102] Table 1.
[0103]
[0104]
[0105]
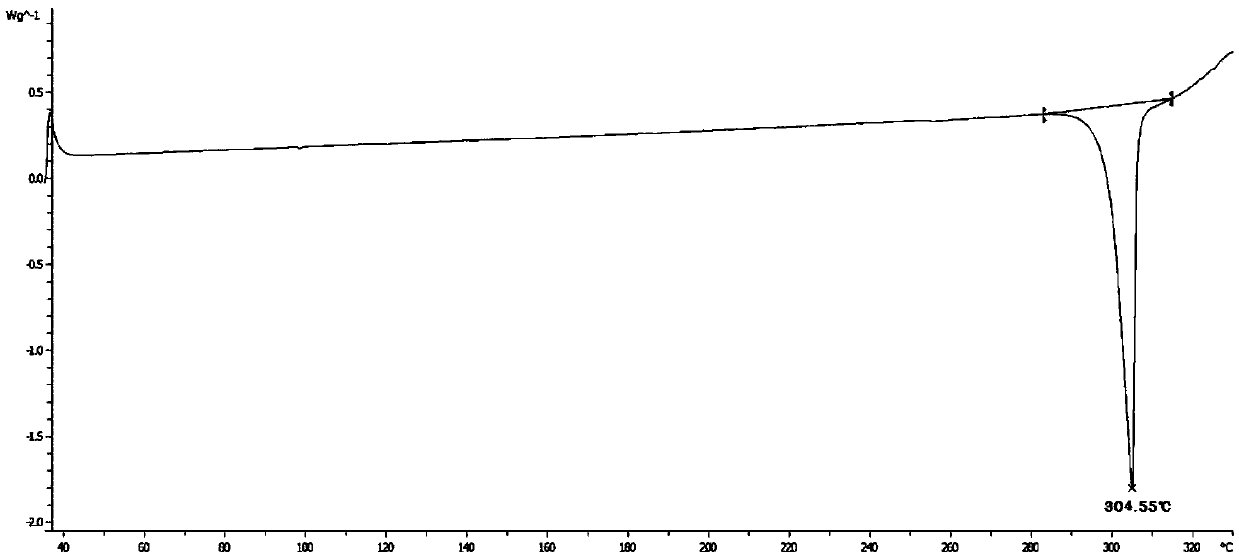
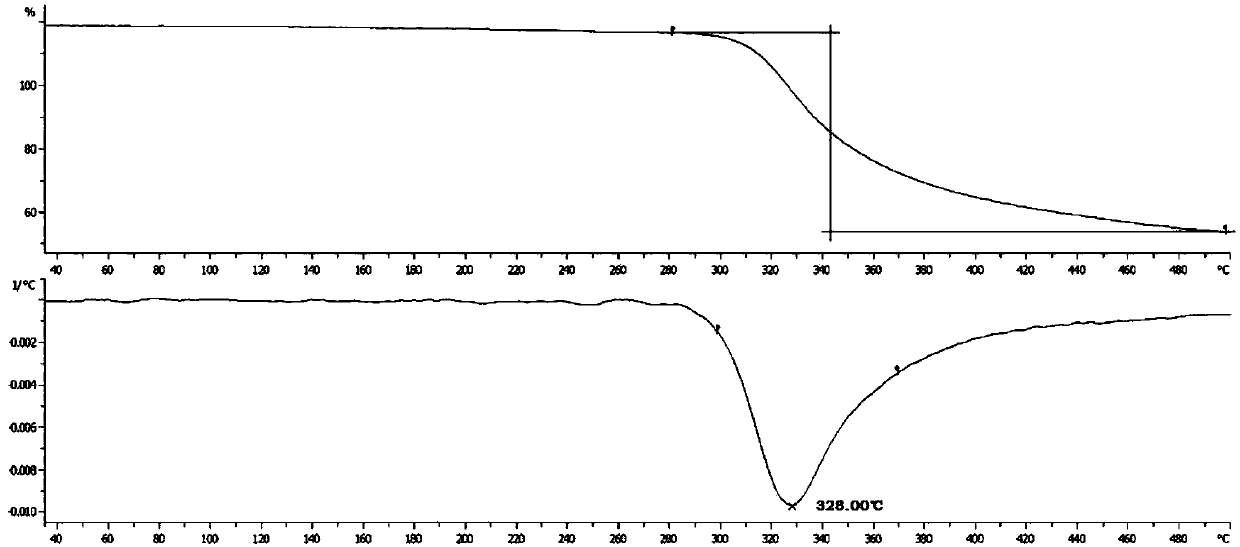
[0106] Differential Scanning Calorim...
Embodiment 3
[0115] Embodiment 3 crystal form I stability test (stability investigation)
[0116] The crystal form I samples were placed in the following two groups of conditions respectively, and samples were taken from two batches before and after the first day and the thirty-first day to detect XRPD.
[0117] group
[0118] Test results: After being placed under the above conditions for 31 days and compared with the test results on the first day, it was found that the XRPD pattern was consistent and the crystal form did not change. It shows that the crystal form I has better stability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com